Chapter 9: Free Radical Substitution Reaction of Alkanes
Answers to Chapter 9 Practice Questions
9.1
Predict the percentage yield of each product for the monochlorination of isobutane by calculation and compare your calculated numbers to the experiment results. Are they consistent?
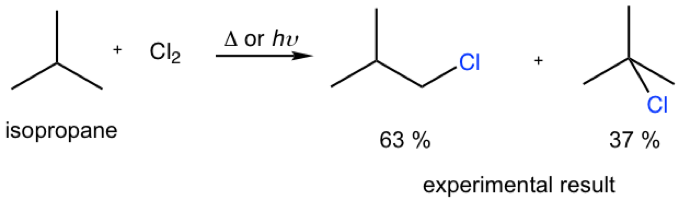
Calculation:
The amount of 1°-chloride: 9 (number of 1°hydrogens) × 1.0 (relative reactivity) = 9.0
The amount of 3°-chloride: 1 (number of 3°hydrogens) × 3.8 (relative reactivity) = 3.8
yield% of 1°-chloride: 8.0/12.8 = 70.3%
yield% of 3°-chloride 3.8/12.8 = 29.7%
The calculated values are consistent with the experiment results, but not exactly the same though.
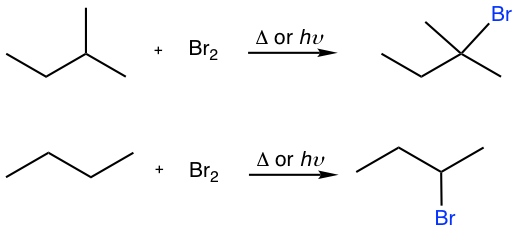
9.2
Show the major bromination product of the following reactions.
9.3
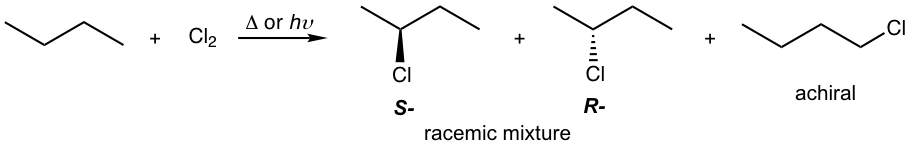
Show all the mono-chlorination products of butane with any stereoisomers when applied.
9.4
Design the synthesis route.


