Chapter 5: Stereochemistry
5.6 Compounds with More Than One Chirality Centers
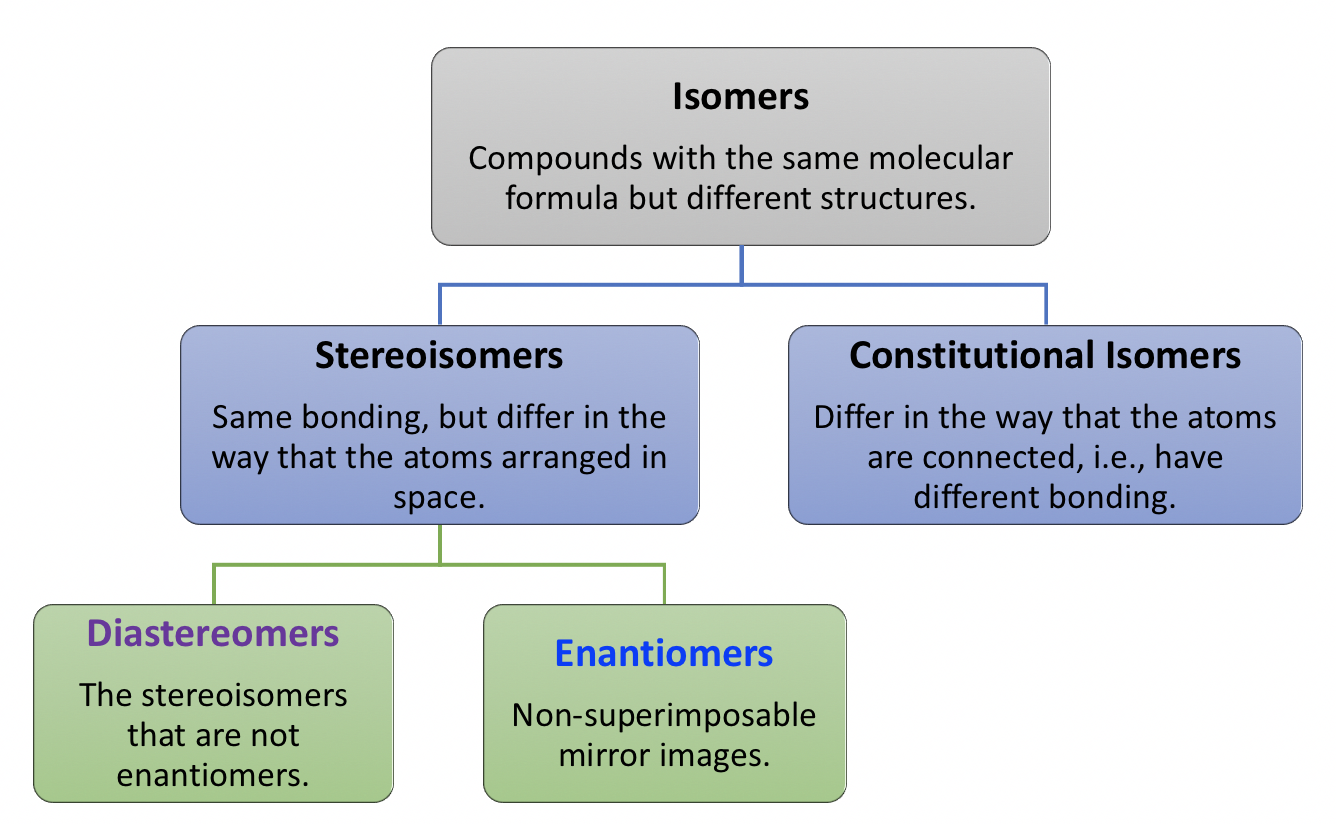
5.6.1 Diastereomers
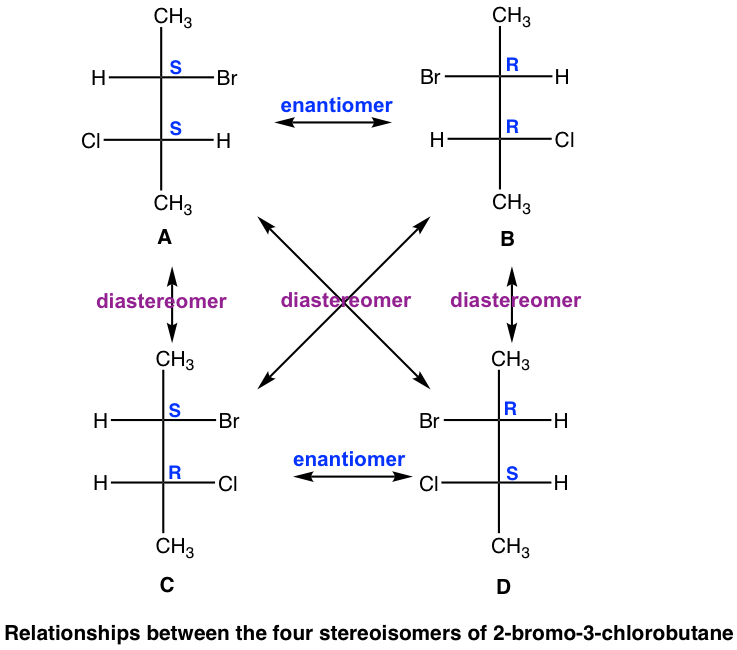
It is very common for there to be more than one chirality centers in an organic compound. For the example of 2-bromo-3-chlorobutane below, there are 2 chirality centers, C2 and C3. As each chirality center has two possible configurations, R and S, the total number of possible stereoisomers for this compound is four, with configurations on C2 and C3 as RR, SS, RS and SR, respectively.
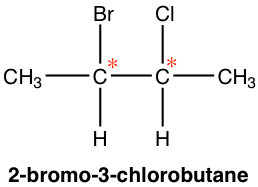
As a general rule, for a compound that has n chirality centers, the maximum number of stereoisomers for that compound is 2n.
The four stereoisomers of 2-bromo-3-chlorobutane consist of two pairs of enantiomers. Stereoisomers A and B are a pair of non-superimposable mirror images, so they are enantiomers. So are the isomers C and D. What then is the relationship between isomers A and C?
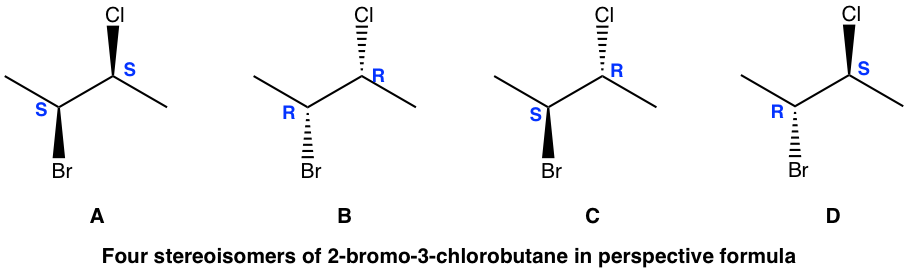
A and C are not identical, not enantiomers, and they are stereoisomers (have the same bonding but differ in the spatial arrangement of groups). This type of stereoisomer is defined as a diastereomer. Diastereomers are stereoisomers that are not enantiomers. For the four stereoisomers here, there are four pairs of diastereomers: A and C, A and D, B and C, and B and D. The relationship between the four stereoisomers can be summarized as:
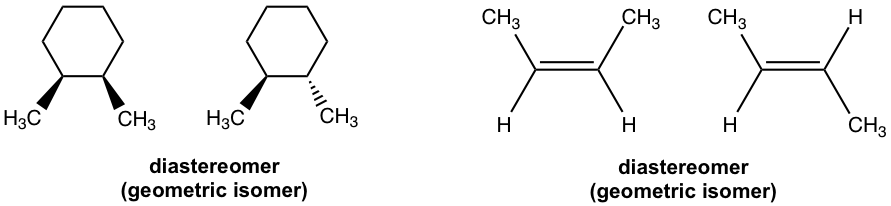
With the introduction of the diastereomer concept, the way to categorize isomers can be revised, and the summary in Fig. 5.1a can be replaced by the updated version in Fig. 5.6a. The stereoisomer then has two sub-types; enantiomers and diastereomers, because any stereoisomers that are not enantiomers can always be called diastereomers. Based on such a definition, the geometric isomers we learned about earlier also belong to the diastereomer category.
As mentioned earlier, enantiomers are very similar to each other, and they share the same physical properties except optical activity (the opposite sign for a specific rotation). Enantiomers also generally have same chemical properties, except for the reaction with other chiral reagents (not topics in this course).
However, diastereomers are not that closely related. Diastereomers have different physical properties, for example, different b.p., color, density, polarity, solubility, etc. They also have different chemical properties.
Next, we will go through examples of cyclic compounds to see how the new concept of the diastereomer relates to the knowledge of cyclic compounds we learned before.
Examples
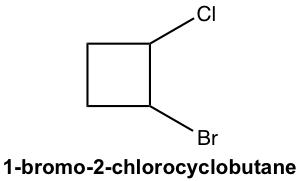
Approach:
There are two chirality centers for a 1-bromo-2-chlorocyclobutane molecule. So, the maximum number of stereoisomers is four. To work on the stereoisomers for cyclic compounds, we can start with a cis/trans isomer and then check whether the enantiomer applies to each case.
Solution:
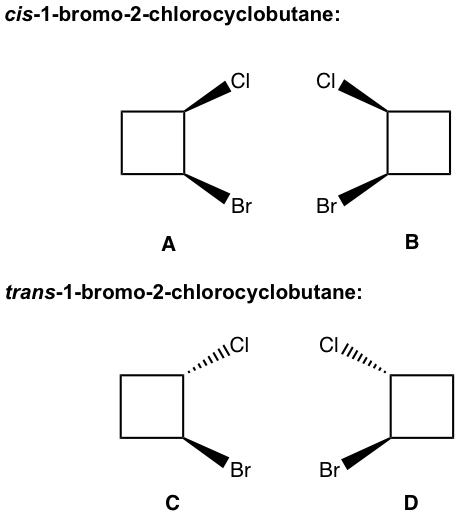
There are two cis-isomers: A and B, and they are enantiomers of each other; similarly, there are also two trans-isomers: C and D that are enantiomers of each other as well.
The relationship between any of the cis-isomers to any of the trans-isomers is diastereomers (A and C, A and D, B and C, and B and D). Since they are geometric isomers, and as we learned earlier geometric isomers can also be called diastereomers.
All geometric isomers are diastereomers (it is always correct to call a pair of geometric isomers diastereomers); however, not all diastereomers are geometric isomers!
Examples:
What is the relationship between the following pair of compounds, enantiomers, identical, diastereomers, constitutional isomers, and non-isomers?
1.
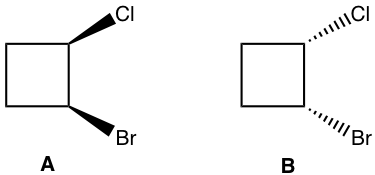
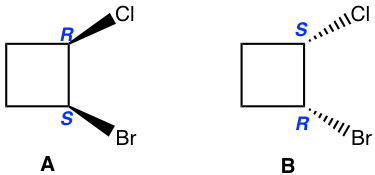
Method I: The basic way is to determine the configuration of each chirality center. As shown below the configuration for both chirality centres are right opposite between the structure A and B. So they are enantiomers.
Method II: For the cyclic structures, sometimes rotating or flipping a given structure in a certain way helps us to tell the relationship (using the molecular model helps the rotate or flip part). For this example, flipping structure B horizontally leads to structure C, B and C are identical. Then it is easy to tell that A and C are just non-superimposable mirror images of each other, so A and C are enantiomers, then A and B are enantiomers as well.
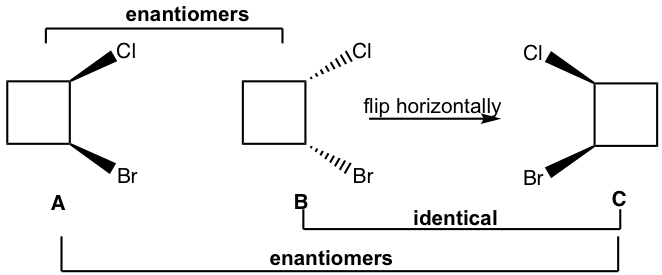
If this method looks confusing to you, then you can stick to Method I.
2.
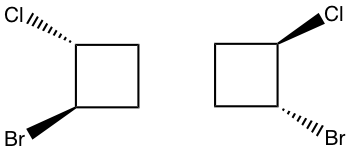
You can use either of the above methods, the answer is “identical”.
5.6.2 Meso compound
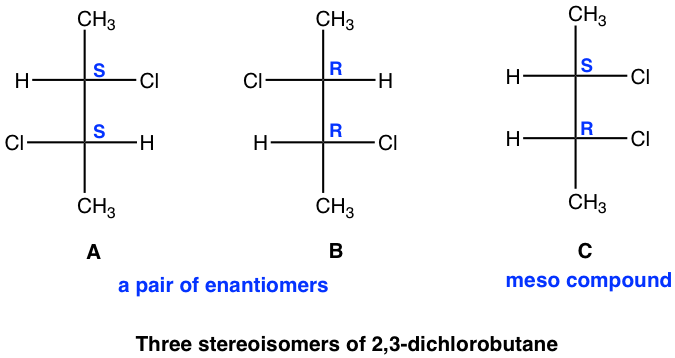
Next, we will see another example of a compound containing two chirality centers, 2,3-dichlorobutane, a compound that has the same substituents on C2 and C3 carbons.
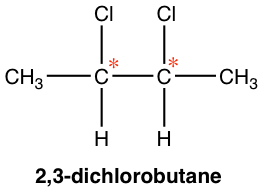
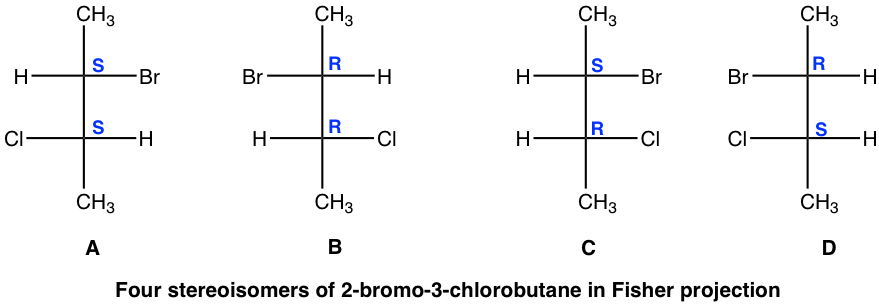
Theoretically, there are a maximum of four stereoisomers, and the structures are shown here by Fisher projections.
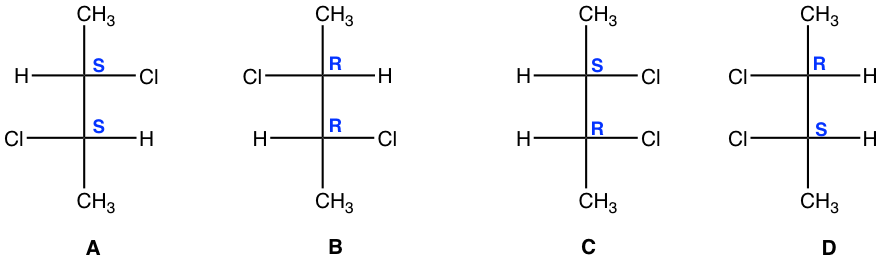
Stereoisomers A and B are non-superimposable mirror images, so they are enantiomers.
We will take a detailed look at stereoisomers C and D. Yes, they are mirror images, but are they non-superimposable? If isomer C is rotated 180° (180° rotation still gets the same structure back for a Fisher projection), then it could get superimposed on isomer D. So, isomer C and D are superimposable mirror images, which means they are the same, identical!
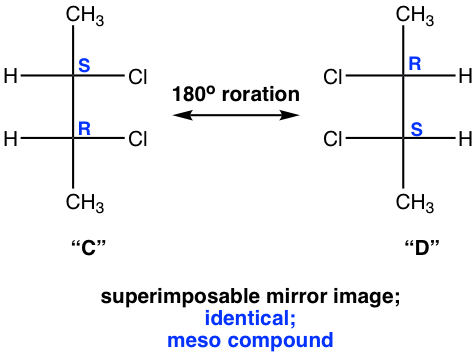
Then “C” and “D” are just different drawings for the same stereoisomer. The next question is whether this stereoisomer is chiral. We have confirmed that this isomer does get superimposed on its mirror image, which means it is achiral.
This is so weird! Can a compound that contains two chirality centers (C2 and C3) be achiral?
Yes, it can! A compound that is achiral but contains chirality centers is called a meso compound. A meso compound is achiral and optical inactive (it does NOT rotate the plane of polarization of plane-polarized light), but it does have multiple chirality centers.
Because that one stereoisomer is a meso compound, the total number of stereoisomers for 2,3-dichlorobutane is three.
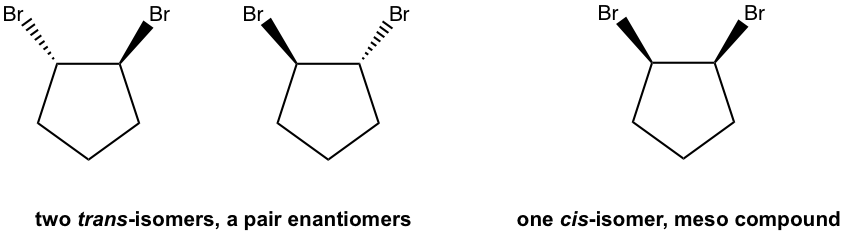
Examples: Draw all the stereoisomers of 1,2-dibromocyclopentane.
Solutions: there are a total of three stereoisomers.
Exercises 5.8
- Draw all stereoisomers for 1-ethyl-3-methylcyclohexane.
- Draw all stereoisomers for 1-ethyl-4-methylcyclohexane.
- Draw all stereoisomers for 1,2-dimethylcyclohexane.
Answers to Chapter 5 Practice Questions
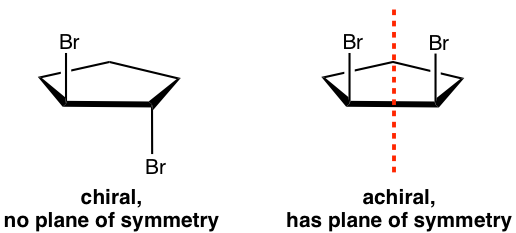
5.6.3 Chiral or achiral by looking for Plane of symmetry
The existence of chirality centers does not guarantee the chirality of a molecule, for example, of the meso compound. Following the definition of chirality always involves the comparison between the original structure and its mirror image, which requires extra work. Is there any easier way to tell whether a molecule is chiral or achiral?
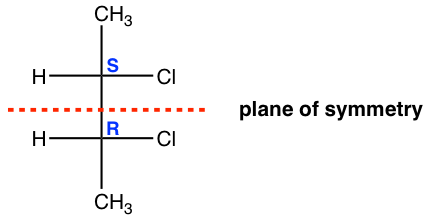
We can check the plane of symmetry. The plane of symmetry is a plane that cuts the molecule in half, and each half is the mirror image of the other.
- If a molecule does have a plan of symmetry, then the molecule is achiral.
- A molecule that does not have a plane of symmetry in any conformation is chiral.
For the meso isomer of 2,3-dichlorobutane, the plane of symmetry is the plane that is labeled in the structure below.
Examples:
Solution:
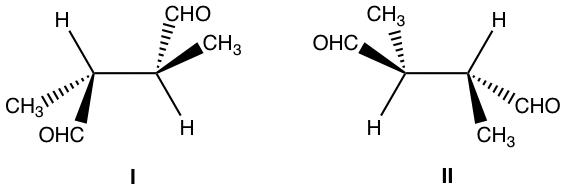
Examples: What is the relationship of the following pair of structures?
Approach: Determine the R/S configuration of each carbon.
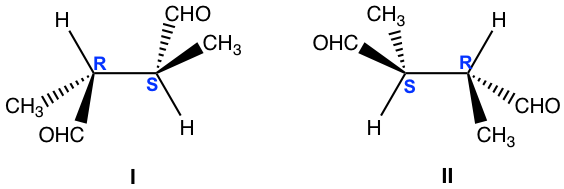
For both structures, the chirality centers are bonded with the same groups, and structure I has R and S, and structure II has S and R. Are they enantiomers?
A bit further investigation is necessary to get the conclusion. Let’s rotate the groups around the 2nd chirality centre of structure I (you can use the molecular model to do the rotation, which is very helpful for visualizing the spatial arrangement of the groups):

Rotation of the groups around the chirality centre does not change the configuration, however, it does change the conformation to eclipsed conformation. In the eclipsed conformation, it is easier to tell that the structure has a plane of symmetry, so it is a meso compound that is achiral. Achiral compound does not have an enantiomer, so structure II is also a meso compound that is identical to the structure I.
Solution: Identical
(You can rotate, or do switches to compare between the two structures, but make sure to keep track of any action. If it is easy to get lost by rotating or switching, assigning R/S configuration is a safer way.)
Examples
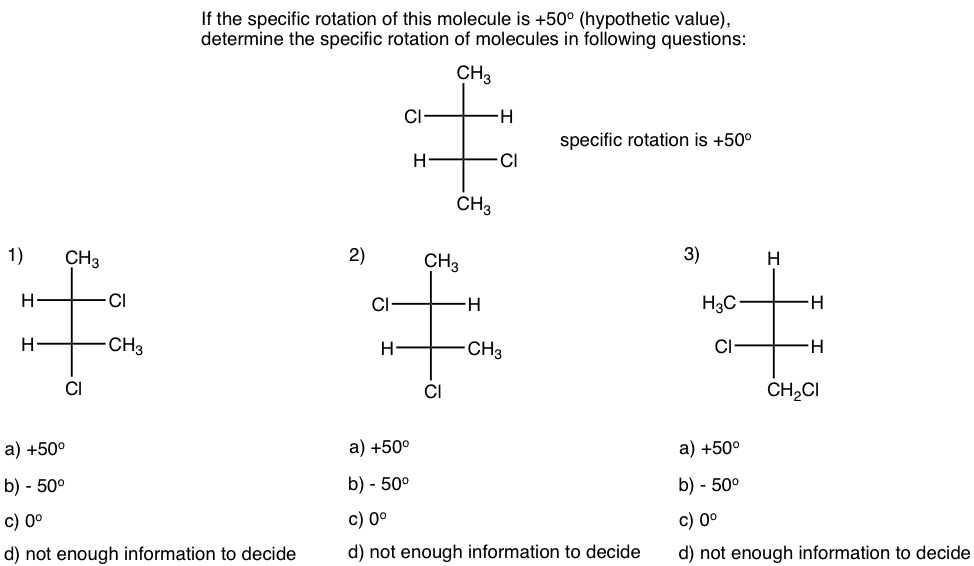
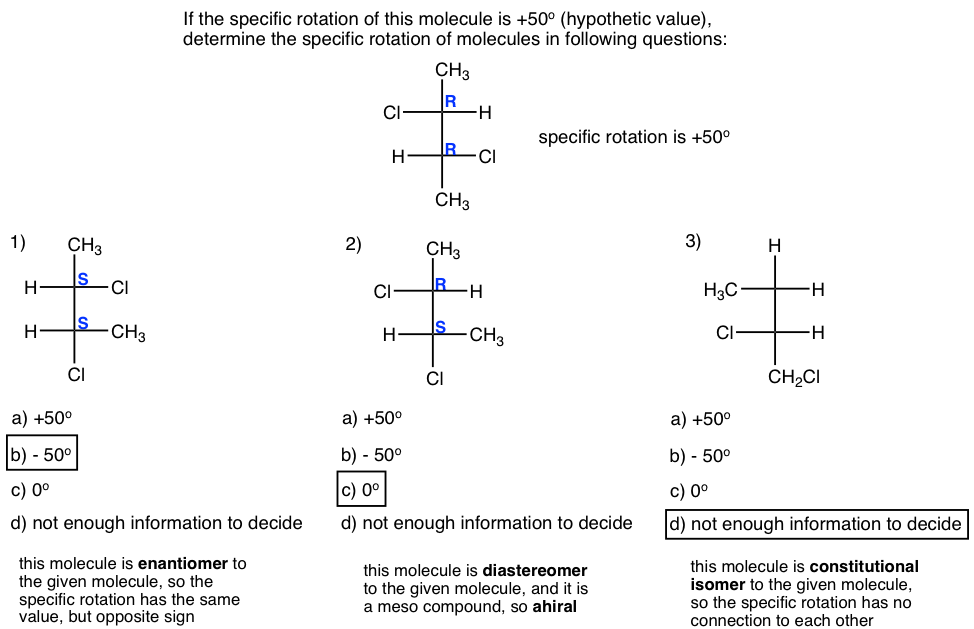
Thinking: Determine the relationship between the molecule in each question with the given one, and apply the knowledge of specific rotation.
Solutions: