Chapter 5: Stereochemistry
Answers to Chapter 5 Practice Questions
5.1 Order the following groups based on decreasing priority for E/Z naming purpose.

Answer: D > C > A > B
5.2
- Draw the structure of the following compounds. Determine which one has an chirality center and label it with a star.
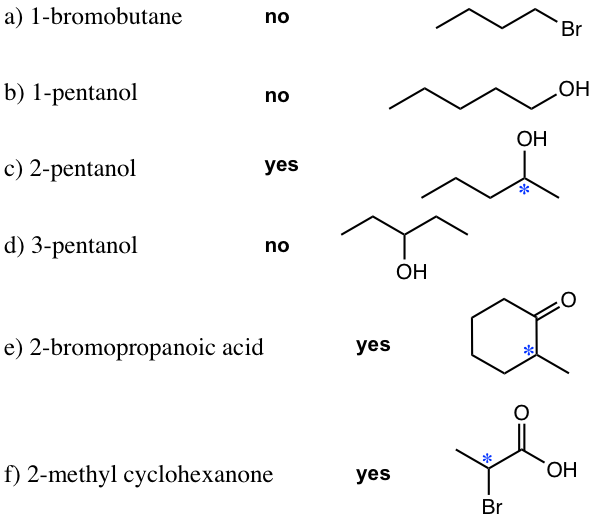
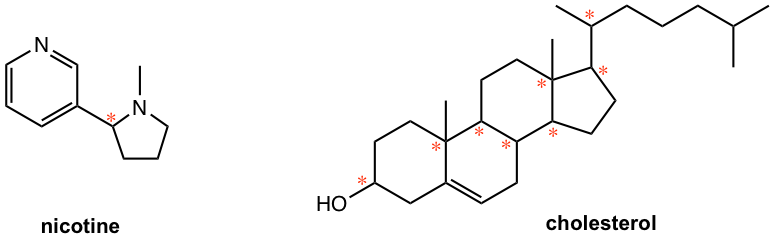
2. Label all the chirality centers in the following molecules.
5.3 Draw the pair of enantiomers of 2-chloro-1-propanol.
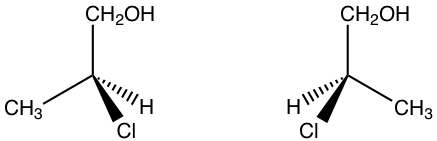
5.4 Determine the R/S configuration of the chirality center in following compounds.

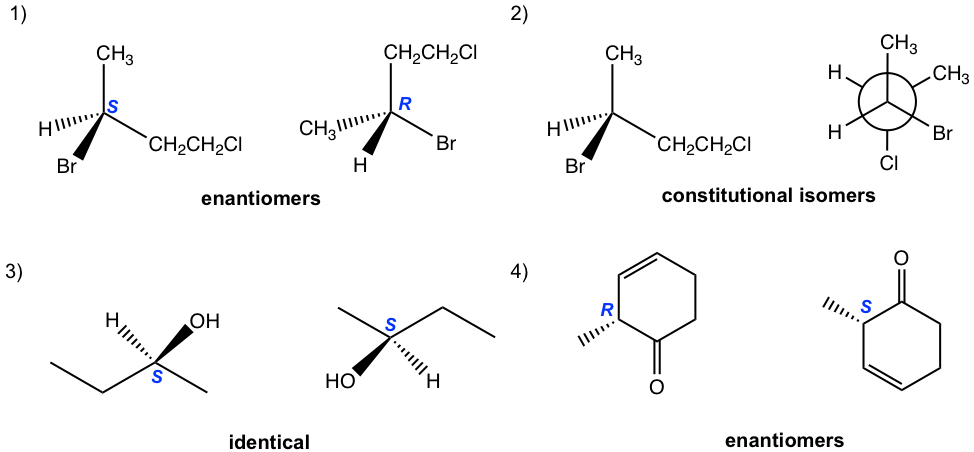
5.6 Draw the diagram for Sample #5 by referring to the diagram for Sample #4.
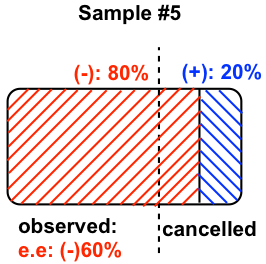
5.7 Explain why, in step 3 of the above procedure, the answer should be reversed to get the final (actual) configuration?
According to the definition of a Fisher projection, the horizontal bond is the bond pointing towards the viewer. Therefore, when the lowest priority group is on a horizontal bond, it is on the position just opposite to the way defined by the Cahn-Ingold-Prelog rule, so the actual configuration should be the reversed version of whatever is initially obtained.
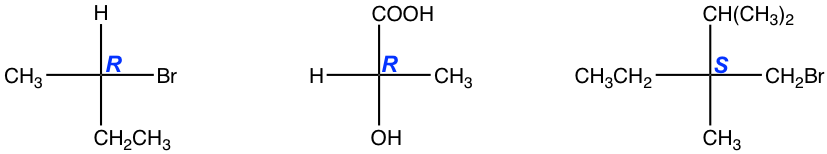
5.9
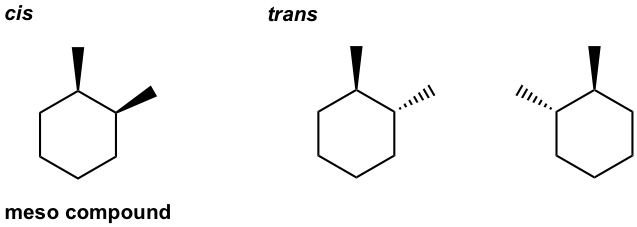
- Draw all stereoisomers for 1-ethyl-3-methylcyclohexane.
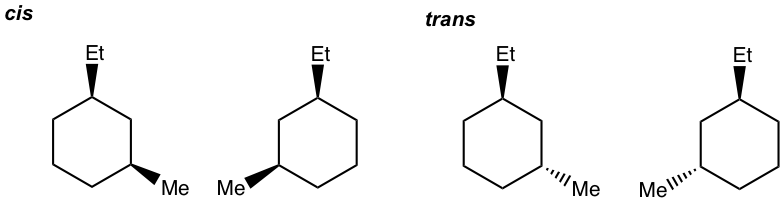
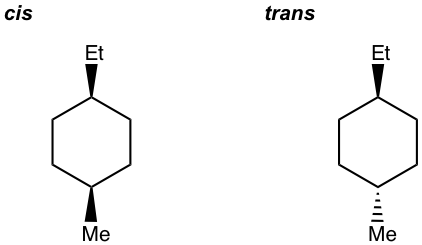
- Draw all stereoisomers for 1-ethyl-4-methylcyclohexane.
- Draw all stereoisomers for 1,2-dimethylcyclohexane.