Chapter 9: Free Radical Substitution Reaction of Alkanes
9.4 Chlorination vs Bromination
9.4.1 Monochlorination
First, we will focus on the monochlorination product by assuming that chlorination only occurs once. Since chlorine is a rather reactive reagent, it shows relatively low selectivity, which means Cl2 does not discriminate greatly among the different types of hydrogen atoms (primary, secondary, or tertiary) in an alkane. As a result, for the reaction of alkane with different hydrogen atoms, a mixture of isomeric monochlorinated products is obtained.
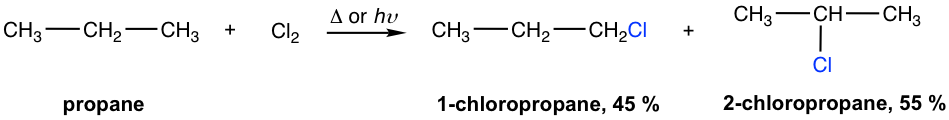
The experimental results of the monochlorination of propane indicate that 45% primary chloride (1-chloropropane) and 55% secondary chloride (2-chloropropane) are produced. How can this result be explained?
To predict the relative amount of different chlorination products, we need to consider two factors at the same time: reactivity and probability.
It has been discussed in section 9.3 that different radicals (primary, secondary or tertiary) have different stability and reactivity. The relative reaction rate of alkyl radicals for chlorination has been measured and has the approximate values of:

Probability simply depends on how many hydrogen atoms there are for each type. With more hydrogen atoms available, the chance for that type of hydrogen to react is statistically higher.
So, the overall amount of each isomeric product should be estimated by accounting for both reactivity and probability; that is:
the amount of a certain type of product = the number of that type of hydrogens × relative reactivity
For the example of the monochlorination of propane, the calculation is:
Amount of 1-chloropropane: 6 (number of 1°hydrogens) × 1.0 (relative reactivity) = 6.0
Amount of 2-chloropropane: 2 (number of 2°hydrogens) × 3.8 (relative reactivity) = 7.6
yield % of 1-chloropropane: 6.0/13.6 = 44%
yield % of 1-chloropropane 7.6/13.6 = 56%
The calculated values are consistent with the experiment results.
Exercises 9.1
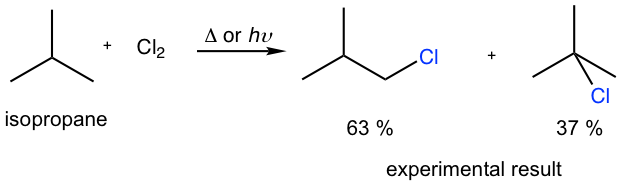
Predict the percentage yield of each product for monochlorination of isobutane by calculation, and compare your calculated numbers to the experiment results. Are they consistent?
Answers to Chapter 9 Practice Questions
For an alkane with only one type of hydrogen, the problem of isomeric mixture can be prevented since only one product is produced. For the following chlorination of cyclopentane, only one monochloride is produced.
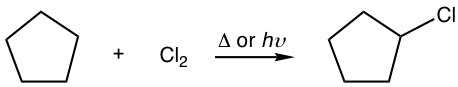
9.4.2 Multichlorination
Although we assume that chlorination occurs once, as the last section discusses, this is not the actual case. A common issue with chlorination is that multiple substitution always happens. A simple example is the chlorination of methane, in which a mixture of multiple chlorination products is obtained, as we learned before.
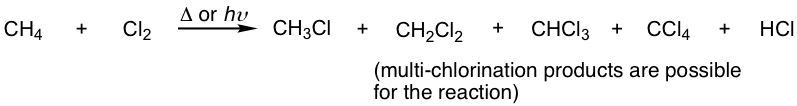
The mechanism for the formation of a multichlorination product is similar to that of monochloride. When chloromethane (or methyl chloride) reacts with Cl2, another hydrogen is replaced by a chlorine atom to give dichloromethane, dichloromethane reacts with Cl2 again to give trichloromethane, and trichloromethane reacts further to produce tetrachloromethane. All the reactions still go through similar propagation steps with the radical mechanism.
Examples
Show the mechanism of propagation steps for the formation of dichloromethane from chloromethane.
Solution:
Practically, to minimize the problem of multichlorination products, the reaction conditions can be controlled in certain ways, for example:
- Use the high concentration of alkane relative to Cl2, to decrease the possibility of multichlorination;
- Control the reaction time: stop the reaction after a “short” time to favor the monochlorination product.
These methods help reduce the number of multichlorination products, but the problem still cannot be completely avoided.
9.4.3 Bromination
Because of the two major problems of chlorination: the lack of selectivity and multi-substitution, chlorination is not useful as a synthesis method to prepare a specific alkyl halide product. Instead, bromination with Br2 can be applied for that purpose. The relative lower reactivity of bromine makes it exhibit a much greater selectivity. Bromine is less reactive, which means it reacts more slowly; therefore, it has the chance to differentiate between the different types of hydrogens, and selectively reacts with the most reactive one. The relative reaction rate of bromination for different radicals is shown here, and you can see the big difference to that of chlorination:

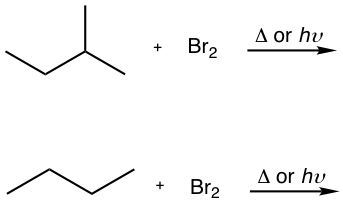
For bromination, the reactivity difference between different types of positions is so high that the reactivity factor becomes predominant for determining the product. Therefore, bromination usually occurs selectively on the most reactive position (the position that forms the most stable carbon radical intermediate) and gives one major product exclusively, as shown in the example here for the bromination of isobutane.
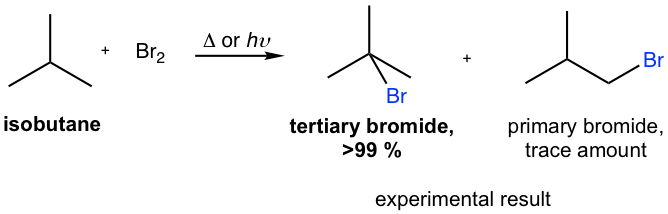
As a result, bromination has the greatest utility synthesis of alkyl halide.
Exercises 9.2
Show the major bromination product of the following reactions.