Chapter 8: Elimination Reactions
Answers to Chapter 8 Practice Questions
8.1 Show the elimination product of the following reactions.
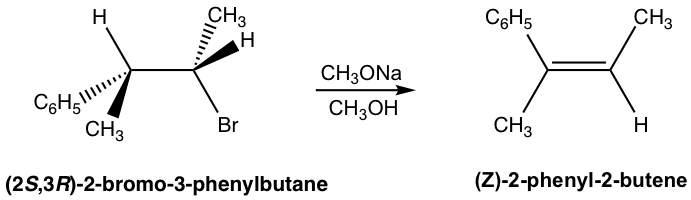
2.
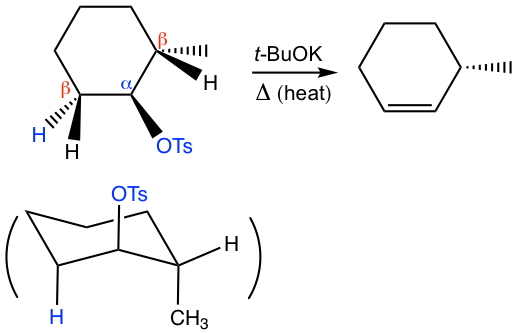
The anti-coplanar conformation of H and leaving group OTs are shown more clearly in the chair conformation of the cyclohexane. Please note that the other β-H can not be anti to the leaving group OTs. Also, to fit to the anti-coplanar requirement, both H and OTs have to be in axial positions so this conformation is the one that undergoes the elimination, though it is not the most stable one. Since the most stable conformation does not fit the E2 stereochemistry requirement, the elimination has to go through the less stable conformation. Heat is preferred to facilitate the reaction.

