Chapter 9: Free Radical Substitution Reaction of Alkanes
9.3 Stability of Alkyl Radicals
The alkyl radical is the key intermediate for the halogenation reaction of alkanes, so the relative stability of radicals determines the relative reactivity. Based on the energy diagram, the alkane that generates the more stable carbon radical exhibits higher reactivity.
The alkyl radicals with different structures show different stabilities. Specifically, tertiary radicals are the most stable, and primary and methyl radicals are the least stable, which follows the same trend as the stability of carbocations.
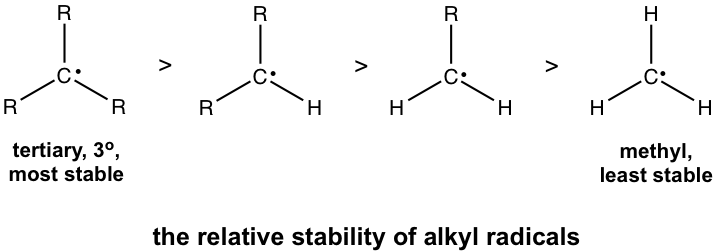
This trend can be explained by two reasons:
- The Hyperconjugation effect of the alkyl (R) group: alkyl groups are electron-donating groups through the hyperconjugation effect (refer to section 7.4), which is the electron density of C-C or C-H σ bond overlap with the half-filled p orbital of carbon radicals. Similar to the carbocation, the carbon radical is also an electron-deficient species, so the electron-donating effect of alkyl groups helps stabilize it. With more alkyl groups involved, the radical is more stable.
- Homolytic bond dissociation energy comparison: Homolytic cleavage of the C–H bond produces carbon radicals. The C–H bond in different structures has different bond dissociation energy. Let’s compare two different types below – primary vs secondary:
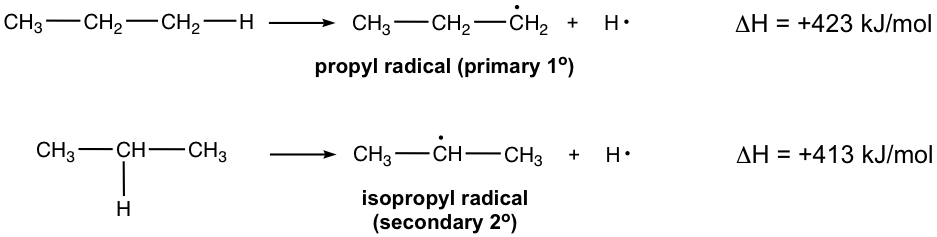
Since both radicals come from the same compound, propane, the higher the homolytic bond dissociation energy, the higher the energy level of the resulting carbon radical. The bond energy of the 1° C–H is 10 kJ/mol higher in energy than the bond energy of the 2° C–H; therefore, the secondary radical is more stable than the primary one.
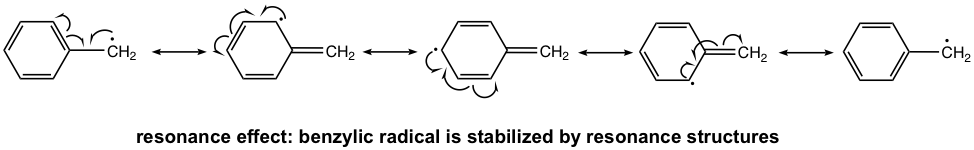
Other than the above reasons, there is another effect that impacts the stability of radicals. For example, the following radical exhibits special stability and is even more stable than other regular tertiary radicals, even though it is a primary radical. Why? This is because of another effect — the resonance effect!
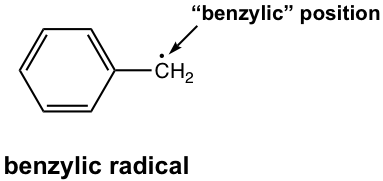
The radical here is not a regular primary radical, as it is on the position beside the benzene ring. The position right next to the benzene ring is called the benzylic position, and this radical is a benzylic radical. Because of the presence of the benzene ring, the benzylic radical has a total of five resonance contributors. According to the resonance effect, the more resonance contributors available, the better the electron density will be dispersed, and the more stable the species will be.
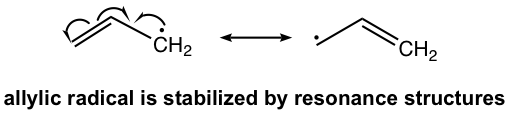
The resonance effect also helps to stabilize the allylic radical. The carbon that is right next to the C=C double bond is the allylic position. The resonance structures of an allylic radical example are shown below. Both benzylic and allylic radicals are more stable than tertiary alkyl radicals because of resonance effects.