Chapter 7: Nucleophilic Substitution Reactions
7.5 SN1 vs SN2
7.5.1 Comparison Between SN1 and SN2 Reactions
As of now, we have finished with the basic concepts of SN1 and SN2 reactions. You have probably already noticed that the two types of reactions have some similarities, but they are also quite different. It will be very helpful to put them together for comparison. To help you get an in-depth understanding of the two types of mechanisms, it is highly recommended that you have a summary in your own way. The following comparison is provided here for your reference.
|
SN1 |
SN2 |
|
| Rate law |
Rate = k[electrophile] |
Rate = k[nucleophile]×[electrophile] |
| Mechanism |
multiple steps with carbocation intermediate |
one step, concerted |
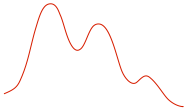
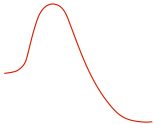
| Reaction Diagram |
|
|
| Stereochemistry |
racemization on reaction center |
inversion on reaction center |
| Electrophilic Substrate | tertiary 3° > secondary 2° > primary 1° and methyl |
primary 1° and methyl > secondary 2° > tertiary 3° |
| Nucleophile |
weak nucleophile, solvolysis |
strong nucleophile |
Table 7.2 Comparison between SN1 and SN2 reactions
7.5.2 Solvent Effect on Sn1 and SN2 Reactions
Other than the factors we have talked about so far, solvents are another key factor that affect nucleophilic substitution reactions. A proper solvent is required to facilitate a certain mechanism. For some cases, choosing the appropriate solvent is an effective way to control on which pathway the reaction proceeds.
To understand the solvent effect, we first need to have more detailed discussions about solvents, then learn how to choose a good solvent for a specific reaction.
Solvents can be divided into three major categories based on the structures and polarities: non-polar, polar protic and polar aprotic solvents.
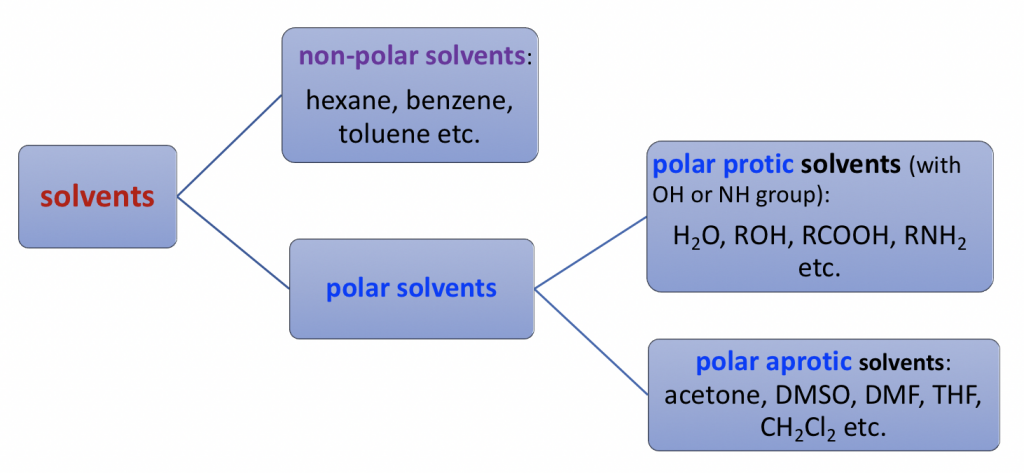
Non-polar solvents are non-polar compounds (hexane, benzene, toluene, etc.)
Polar protic solvents are compounds containing OH or NH groups that are able to form hydrogen bonds. Polar protic solvents are highly polar because of the OH or NH group.
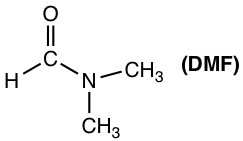
Polar aprotic solvents are group solvents with a medium range of polarity. They are polar because of polar bonds like C=O or S=O, but the polarity is not as high as the OH or NH group. Typical examples of polar aprotic solvents include acetone, DMSO, DMF, THF, and CH2Cl2.
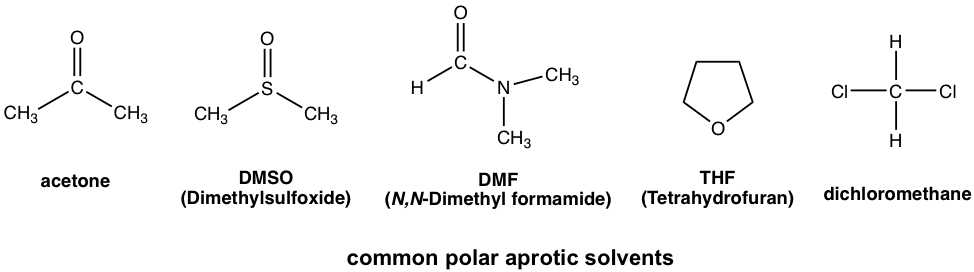
The general guideline for solvents regarding the nucleophilic substitution reaction is:
- SN1 reactions are favored by polar protic solvents (H2O, ROH, etc.), and usually are solvolysis reactions.
- SN2 reactions are favored by polar aprotic solvents (acetone, DMSO, DMF, etc.).
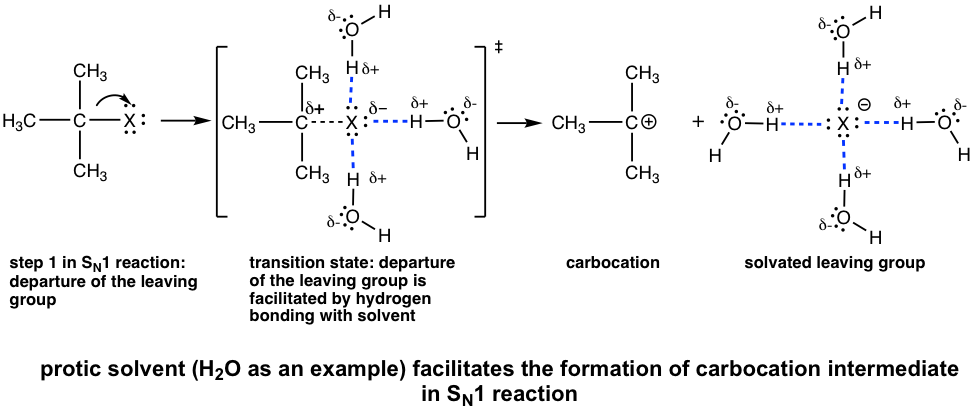
Polar Protic Solvents Favor SN1 Reactions
In an SN1 reaction, the leaving group leaves and a carbocation is formed in the first step, which is also the rate-determining step. The polar solvent, such as water, and MeOH, can form hydrogen bonding with the leaving group in the transition state of the first step, thereby lowering the energy of the transition state that leads to the carbocation and speeding up the rate-determining step. As a result, polar protic solvents facilitate SN1 reactions. It is very common that polar protic solvents also serve as nucleophiles for SN1 reactions so SN1 reactions are usually solvolysis reactions, as we learned earlier.
Polar Aprotic Solvents Favor SN2 Reactions
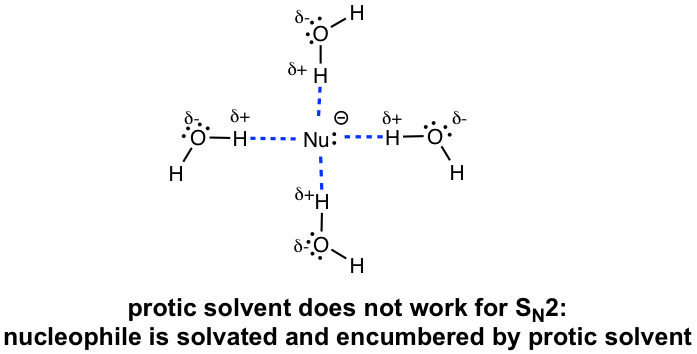
Strong nucleophiles are required in SN2 reactions, and strong nucleophiles are usually negatively charged species, such as OH–, CH3O–, and CN–. These anions must stay with cations in a salt format like NaOH or CH3ONa. Since salts are insoluble in a non-polar solvent, non-polar solvents are not appropriate choices, and we need polar solvents that can dissolve the salts.
The issue for polar protic solvents is that the nucleophile anions will be surrounded by a layer of solvent molecules with hydrogen bonds, and this is called the solvation effect. The solvation effect stabilizes (or encumbers) the nucleophiles and hinders their reactivities in an SN2 reaction. Therefore, polar protic solvents are not suitable for SN2 reactions.
As a result, polar aprotic solvents, such as acetone and DMSO are the best choice for SN2 reactions. They are polar enough to dissolve the salt format nucleophiles and they also do not interact as strongly with anions to hinder their reactivities. The nucleophile anions still move around freely in polar aprotic solvents to act as nucleophiles.
The reaction rates for SN2 reactions in different solvents are provided in Table 7.3 below, and the polar aprotic solvent DMF proved to be the best choice that speeds up the reaction significantly.
|
reaction: CH3I + Cl– → CH3Cl + I– |
|
|
solvent |
relative rate |
|
CH3OH |
1 |
|
|
12.5 |
|
|
1,200,000 |
With all the knowledge we have learned about SN1, SN2 reactions and reaction conditions, we should be able to determine whether a given reaction goes with the SN1 or SN2 pathway or design a proper reaction that will produce the desired product(s). The reaction pathway predominantly depends on the nature of the substrates (primary, secondary, or tertiary), and the choice of proper reaction condition serves as a way to facilitate the process.
- Primary and methyl substrates predominantly undergo SN2 reactions.
- Tertiary substrates go with the SN1 process.
- The reaction of secondary substrates mainly relies on the conditions applied. The conditions include nucleophiles, solvents, etc.
Exercises 7.4
Show the product(s) of the following reactions:
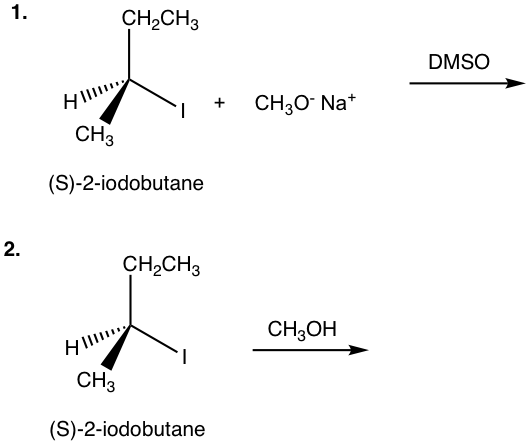
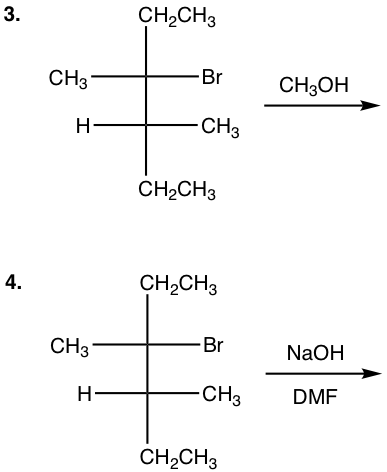
Answers to Chapter 7 Practice Questions
Some practical tips for working on SN1, and SN2 reactions:
- As we understand, strong nucleophiles are required for SN2 reactions, and most of the strong nucleophiles are those with negative charges, for example, OH– and OR–. These nucleophiles can be either shown as anions OH–, CH3O–, C2H5O–, or in a salt format like NaOH, KOH, CH3ONa, or C2H5ONa in the reaction conditions. You should understand that it is the same thing. The anion format is easy to identify and also highlights the nature of these species; however, since anions must stay together with counter cations as salt, the salt format shows the actual chemical formula of the compound used in the reaction.
- Since a polar aprotic solvent favors SN2 reactions, any of the above anions or salt can be used together with DMSO, DMF, etc., such as OH–/DMSO or CH3ONa/DMF. However, sometimes you may see a combination like CH3ONa/CH3OH, which is the combination of CH3O– together with its conjugate acid CH3OH. It may seem contradictory, so why is a strong nucleophile for SN2 combined with a solvent for SN1? The reality is that CH3ONa here still acts as a strong nucleophile and can be used for an SN2 reaction, and CH3OH is the solvent for CH3ONa. The reason why CH3OH is used together as a solvent is that the CH3ONa can be prepared by treating an alcohol with Na. For example:
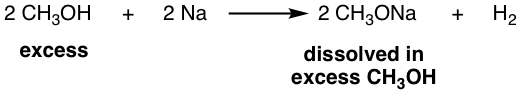
Other alcohols can also react with Na metal (or potassium metal, K) to generate the corresponding RONa.
The reaction between alcohol and NaH can be used as well.
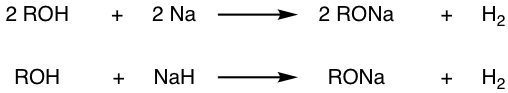 Since alcohol is in excess in the above reactions, it is also a good solvent for the resulting alkoxide, and an RO–/ROH combination is used commonly together. The RO– in this combination can be used as a strong nucleophile for an SN2 reaction or a base in an elimination reaction (Chapter 8).
Since alcohol is in excess in the above reactions, it is also a good solvent for the resulting alkoxide, and an RO–/ROH combination is used commonly together. The RO– in this combination can be used as a strong nucleophile for an SN2 reaction or a base in an elimination reaction (Chapter 8).