Chapter 8: Elimination Reactions
8.2 E1 Reactions
E1 Mechanism
Similar to substitutions, some elimination reactions show first-order kinetics. These reactions go through the E1 mechanism, which is the multiple-step mechanism that includes the carbocation intermediate.
When t-butyl bromide reacts with ethanol, a small amount of elimination products is obtained via the E1 mechanism.
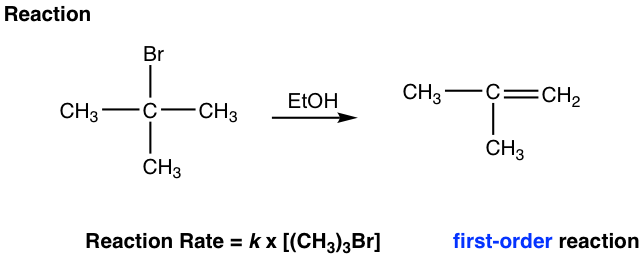
The overall elimination involves two steps:
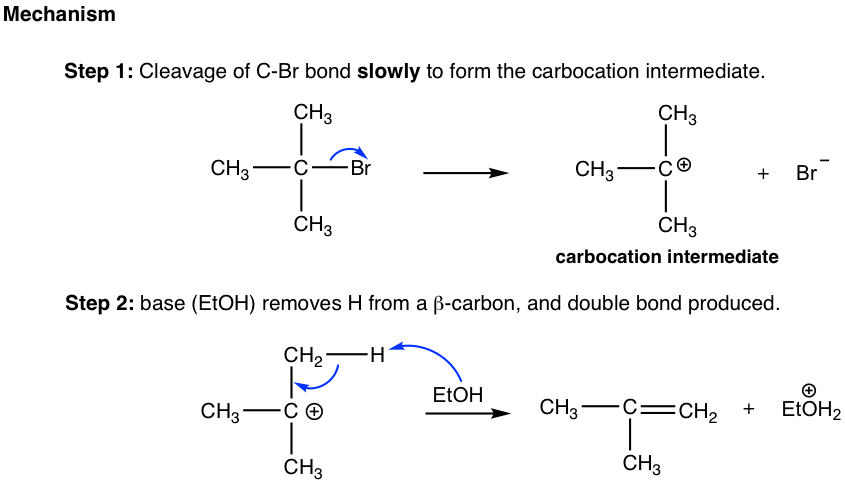
Step 1: The bromide dissociates and forms a tertiary (3°) carbocation. This is a slow bond-breaking step, and it is also the rate-determining step for the whole reaction. Since only the bromide substrate was involved in the rate-determining step, the reaction rate law is first order. This is the reaction rate only depends on the concentration of (CH3)3Br and has nothing to do with the concentration of the base, ethanol.
Step 2: The hydrogen on β-carbon (β-carbon is the one beside the positively charged carbon) is acidic because of the adjacent positive charge. The base, EtOH, reacts with the β-H by removing it, and the C-H bond electron pair moves in to form the C-C π bond.
The base ethanol in this reaction is a neutral molecule and therefore a very weak base. Since a strong base favors E2, a weak base is a good choice for E1 by discouraging it from E2. Ethanol acts as the solvent as well, so the E1 reaction is also a solvolysis reaction.
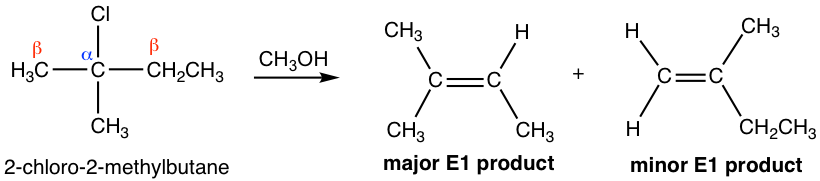
For the E1 reaction, if more than one alkene can be possibly formed as products, the major product will also be the more substituted alkene, like E2, because of the stability of those alkenes.
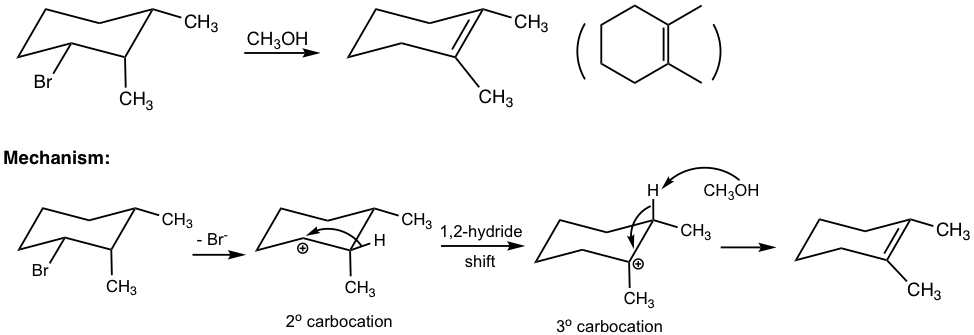
Since the E1 reaction involves a carbocation intermediate, the carbocation rearrangement might occur if such a rearrangement leads to a more stable carbocation. For the following example, the initially formed secondary carbocation undergoes a 1,2-methanide shift to give the more stable tertiary benzylic carbocation, which leads to the final elimination product.
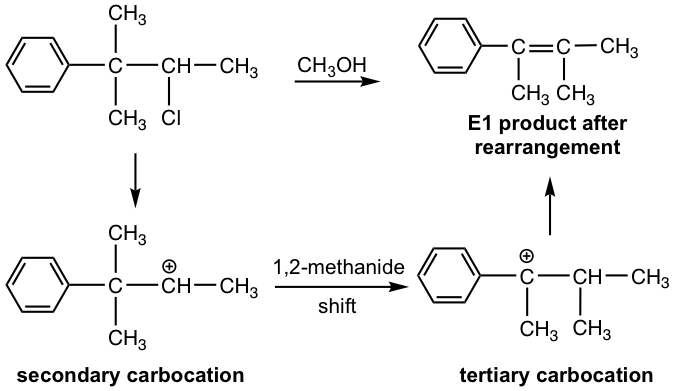
Examples: Show elimination product of the following reaction.
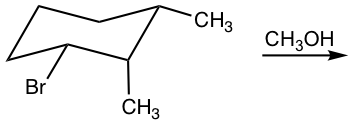
Answer: