Chapter 6: Structural Identification of Organic Compounds: IR and NMR Spectroscopy
6.3 IR Spectrum and Characteristic Absorption Bands
With a basic understanding of IR theory, we will now take a look at the actual output from IR spectroscopy experiments and learn how to get structural information from the IR spectrum. Below is the IR spectrum for 2-hexanone.
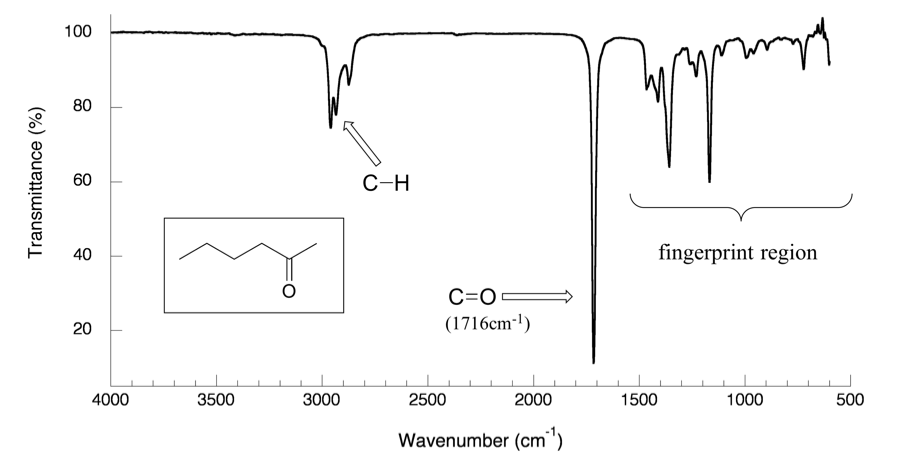
Notes for interpreting IR spectra:
- The vertical axis is ‘% transmittance’, which indicates how strongly light was absorbed at each frequency. The solid line traces the values of % transmittance for every wavelength passed through the sample. At the high end of the axis, 100% transmittance means no absorption occurred at that frequency. Lower values of % transmittance mean that some of the energy is absorbed by the compound and gives downward spikes. The spikes are called absorption bands in the IR spectrum. A molecule has a variety of covalent bonds, and each bond has different vibration modes, so the IR spectrum of a compound usually shows multiple absorption bands.
- The horizontal axis indicates the position of an absorption band, but instead of using frequency to show the absorbed radiation, wavenumbers (
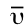 , in the unit of cm-1) are used as a conventional way in IR spectra. The wavenumber is defined as the reciprocal of wavelength (Formula 6.3), and the wavenumbers of IR radiation are normally in the range of 4000 cm-1 to 600 cm-1 (approximately corresponds to the wavelength range of 2.5 μm to 17 μm of IR radiation).
, in the unit of cm-1) are used as a conventional way in IR spectra. The wavenumber is defined as the reciprocal of wavelength (Formula 6.3), and the wavenumbers of IR radiation are normally in the range of 4000 cm-1 to 600 cm-1 (approximately corresponds to the wavelength range of 2.5 μm to 17 μm of IR radiation).
Please note that the direction of the horizontal axis (wavenumber) in IR spectra decreases from left to right. The larger wavenumbers (shorter wavelengths) are associated with higher frequencies and higher energy.
Stretching Vibrations
Generally, stretching vibrations require more energy and show absorption bands in the higher wavenumber/frequency region. The characteristics of stretching vibration bands associated with the bonds in some common functional groups are summarized in Table 6.1.
| Formula |
Bond |
Characteristic IR Frequency range (cm-1) |
| alcohol |
O-H stretching |
3200-3600 (broad)
|
| carbonyl |
C=O stretching |
1650-1750 (strong)
|
| aldehyde |
C-H stretching |
~ 2800 and ~ 2700 (medium)
|
| carboxylic acid |
C=O stretching |
1700-1725 (strong)
|
|
O-H stretching |
2500-3300 (broad)
|
|
| alkene |
C=C stretching |
1620-1680 (weak)
|
|
vinyl =C-H stretching |
3020-3080
|
|
| benzene |
C=C stretching |
~ 1600 and 1500-1430
(strong to weak)
|
| alkyne |
C≡C stretching |
2100-2250 (weak)
|
|
terminal ≡C-H stretching |
3250-3350
|
|
| alkane |
C-H stretching |
2850-2950
|
| amine |
N-H stretching |
3300-3500 (medium)
|
Table 6.1 Characteristic IR Frequencies of Stretching Vibrations
The information in Table 6.1 can be summarized in the diagram for easier identification (Figure 6.3b), in which the IR spectrum is divided into several regions, with the characteristic band of certain groups labeled.
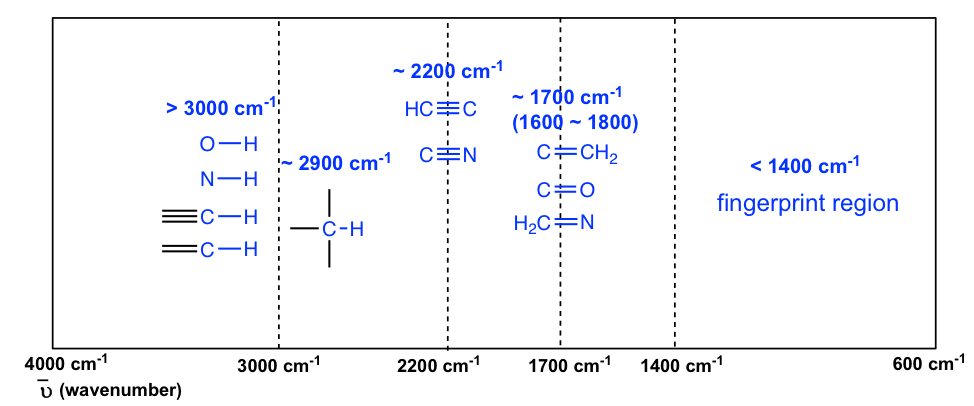
The absorption bands in IR spectra have different intensities that can usually be referred to as strong (s), medium (m), weak (w), broad and sharp. The intensity of an absorption band depends on the polarity of the bond, and a bond with a higher polarity will show a more intense absorption band. The intensity also depends on the number of bonds responsible for the absorption, and an absorption band with more bonds involved has a higher intensity.
The polar O-H bond (in alcohol and carboxylic acid) usually shows strong and broad absorption bands that are easy to identify. The broad shape of the absorption band results from the hydrogen bonding of the OH groups between molecules. The OH bond of an alcohol group usually has absorption in the range of 3200–3600 cm-1, while the OH bond of the carboxylic acid group occurs at about 2500–3300 cm-1 (Figure 6.4a and Figure 6.4c).
The polarity of the N-H bond (in amine and amide) is weaker than the OH bond, so the absorption band of N-H is not as intense or as broad as O-H, and the position is in the 3300–3500 cm-1 region.
The C-H bond stretching of all hydrocarbons occurs in the range of 2800–3300 cm-1, and the exact location can be used to distinguish between alkane, alkene and alkyne. Specifically:
- ≡C-H (sp C-H) bond of terminal alkyne gives absorption at about 3300 cm-1
- =C-H (sp2 C-H) bond of alkene gives absorption at about 3000-3100 cm-1
- -C-H (sp3 C-H) bond of alkane gives absorption at about ~2900 cm-1 (see the example of the IR spectrum of 2-hexanone in Figure 6.3a; the C-H absorption band at about 2900 cm-1)
A special note should be made for the C-H bond stretching of an aldehyde group that shows two absorption bands: one at ~2800 cm-1 and the other at ~ 2700 cm-1. It is therefore relatively easy to identify the aldehyde group (together with the C=O stretching at about 1700 cm-1) since essentially no other absorptions occur at these wavenumbers (see the example of the IR spectrum of butanal in Figure 6.4d).
The stretching vibration of triple bonds C≡C and C≡N have absorption bands of about 2100–2200 cm-1. The band intensity is at a medium to weak level. The alkynes can generally be identified with the characteristic weak but sharp IR absorbance bands in the range of 2100–2250 cm-1 due to stretching of the C≡C triple bond, and terminal alkynes can be identified by their absorbance at about 3300 cm-1 due to stretching of sp C-H.
As mentioned earlier, the C=O stretching has a strong absorption band in the 1650–1750 cm-1 region. Other double bonds like C=C and C=N have absorptions in lower frequency regions of about 1550–1650 cm-1. The C=C stretching of an alkene only shows one band at ~1600 cm-1 (Figure 6.4b), while a benzene ring is indicated by two sharp absorption bands: one at ~1600 cm-1 and one at 1500–1430 cm-1 (see the example of the IR spectrum of ethyl benzene in Figure 6.4e).
You will notice in Figures 6.3a and 6.3b that a region with a lower frequency of 400~1400 cm-1 in the IR spectrum is called the fingerprint region. Similar to a human fingerprint, the pattern of absorbance bands in the fingerprint region is characteristic of the compound as a whole. Even if two different molecules have the same functional groups, their IR spectra will not be identical, and such a difference will be reflected in the bands in the fingerprint region. Therefore, the IR from an unknown sample can be compared to a database of IR spectra of known standards to confirm the identification of the unknown sample.

