Chapter 3: Acids and Bases: Introduction to Organic Reaction Mechanism Introduction
3.1 Review of Acids and Bases and Ka
The most commonly applied definition of acids and bases is the Brønsted-Lowry definition:
- Brønsted-Lowry Acid: a substance that can donate a proton (H+)
- Brønsted-Lowry Base: a substance that can accept a proton (H+)
Therefore, according to the Brønsted-Lowry definition, an acid-base reaction is a proton transfer process in which the acid gives away a proton and the base accepts a proton as shown in the general equation:
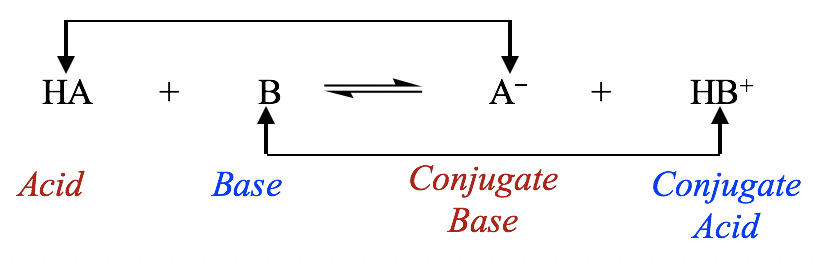 General equation for acid-base reaction
General equation for acid-base reaction
The species that forms when an acid loses its proton is called the conjugate base of that acid; similarly, the species that forms when a base accepts a proton is called the conjugate acid of that base. In the general equation above, HA is the conjugate acid of A–, and A– is the conjugate base of HA. HA and A– can also be called a conjugate acid-base pair; another pair is HB+ and B.
A strong acid donates the proton completely, and the arrow “→” can be used in the reaction equation to indicate that the reaction goes to completion. The dissociation reaction of the strong acid HCl in water is used as an example here:
HCl (g) + H2O (l) →H3O+(aq) + Cl–(aq)
For weak acids (HA is used as a general formula), the proton is only partially donated and the reaction stays at equilibrium. The equilibrium arrow “![]() ” will be needed in the reaction equation to indicate the equilibrium status:
” will be needed in the reaction equation to indicate the equilibrium status:
![]() The equilibrium constant for the above reaction is called the acid dissociation constant, Ka. It is a constant to measure the relative strength of an acid. The expression for Ka is:
The equilibrium constant for the above reaction is called the acid dissociation constant, Ka. It is a constant to measure the relative strength of an acid. The expression for Ka is:
![Ka = [H3O+][A-]/[HA]](https://kpu.pressbooks.pub/app/uploads/sites/139/2020/10/Ka-constant.png)
The larger the Ka value, the stronger the ability of the acid to donate protons, and the stronger the acid is. (Technically, when the Ka value is larger than 10, the acid can be regarded as a strong acid.)

