Chapter 5: Stereochemistry
5.2 Geometric Isomers and the E/Z Naming System
Geometric Isomers of Alkenes
In the discussions about 1,2-dimethylcyclohexane in Chapter 4, we learned that there are two geometric isomers possible for that compound: cis and trans. The restricted C-C bond rotation of the cyclic structure results in the cis or trans isomer of 1,2-dimethylcyclohexane. Restricted rotation also can be caused by a double bond, so geometric isomers apply to some alkenes as well.
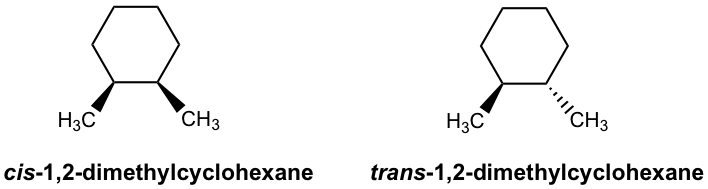
For the example of 2-butene, the condensed structural formula CH3-CH=CH-CH3 does not represent the trigonal planar shape of the sp2 carbons with double bonds. To show the shape explicitly, we need to draw a Kekulé structure that shows all the bond angles. Then, it will be noted that there are two different shapes of 2-butene, with the CH3 groups on either the same side or the opposite side of the double bond.

They are geometric isomers and can be labeled as cis or trans in a similar way as disubstituted cycloalkane. Cis/trans is the common designation for geometric isomers and might be ambiguous for some structures. Here, we will learn the IUPAC naming system for the geometric isomers of alkene, which is the E/Z naming system.
E/Z Naming System
To do the E/Z designation, first, the groups connected on each sp2 double bond carbon will be assigned priority based on the atomic number (see the following guidelines for details). Then, the isomer with the same priority group on the same side of the double bond is assigned “Z”, and the isomer with the same priority group on the opposite side of the double bond is called “E”. Both E and Z come from German: “Zusammen” means “same side” and “Entgegen” means “opposite”.
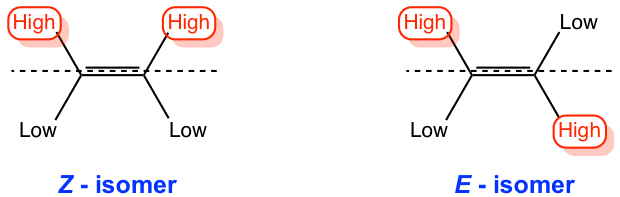
The guidelines for assigning group priority in E/Z naming system
1. Priority is assigned based on the atomic number of the atoms bonded directly to the sp2 double bond carbon – the larger the atomic number, the higher the priority (isotopes with a higher mass number have higher priority). For example: S > O > N > C > H.
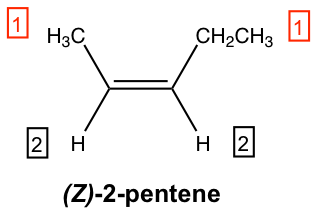
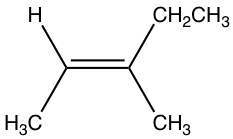
For the above structure of 2-penetene: on the left-side sp2 carbon, the methyl group CH3 is higher than the hydrogen atom because C > H; on the right-side sp2 carbon, the ethyl group CH2CH3 is also higher than hydrogen. With higher priority groups on both sides of the double bond, this is the Z isomer, and the complete name of the compound is (Z)-2-pentene.
The group with higher priority is labelled as #1, and the group with lower priority is labelled as #2 in this book.
2. If the two groups bonded directly on an sp2 carbon start with the same atom, it means there is a tie from step 1. Then, we move on to the atoms connected to the “tied” atom, and priority increases as the atomic number of the next attached atom increases.
 For the above structure, it is obvious that Cl is higher than C (C of CH2CH3 group) on the right-side sp2 carbon.
For the above structure, it is obvious that Cl is higher than C (C of CH2CH3 group) on the right-side sp2 carbon.
On the left-side sp2 carbon, we need to compare between the methyl CH3 group and ethyl CH2CH3 group. Both groups have carbon atoms attached directly to the sp2 carbon, which is a tie. In the CH3 group, the carbon atom is bonded to H, H, H, while in the CH2CH3 group the carbon atom is bonded with H, H, C. So, ethyl CH2CH3 is higher than methyl CH3 (see the Note below). Since the higher priority groups are on the opposite side of the double bond, this is the E isomer, and the complete name of the compound is (E)-3-chloro-4-methyl-3-hexene.
Note #1: For this round of comparison between H, H, H and H, H, C, compare the single atom with the greatest number in one group versus the single atom with the greatest number in the other group. So, it is H in one group versus C in the other group, since C > H; therefore, CH2CH3 is higher than CH3. Remember, do not add the atomic numbers. For example, if one group has C, C, C, and the other group has C, O, H, then the C, O, H side is higher because O is higher than C.
Note #2: The above compound is a cis-isomer if using the cis/trans naming system (both ethyl groups are on the same side of the double bond), but it is an E-isomer for the E/Z system. So, cis/trans and E/Z are two different naming systems, but they do not always match.
3. Repeat step 2 if necessary until the priority is assigned.
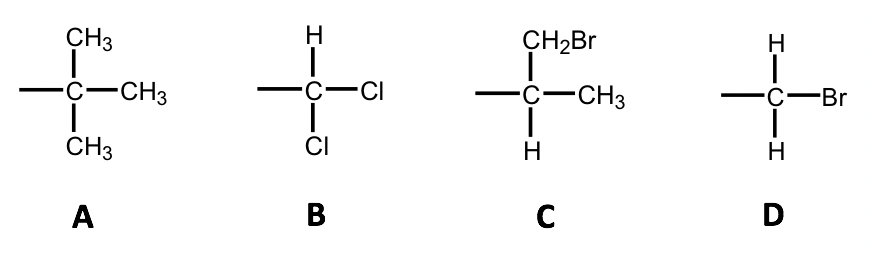
Examples: What is the correct structural formula of (E)-2-bromo-3-chloro-2-butene?
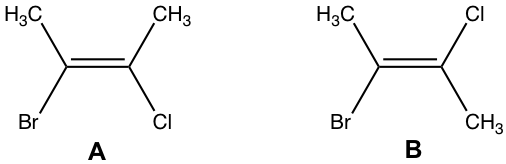
The answer is B.
Examples: Draw the structure of (E)-3-methyl-2-pentene
Answer:
Examples: Order the following groups based on increasing priority.
Approach:
1st round: C, C, C, C (tie);
2nd round:
A: C bonded to C, C, C; (3rd)
B: C bonded to H, Cl, Cl; (Cl is the 2nd high)
C: C bonded to H, C, C; (4th)
D: C bonded to H, H, Br (Br is the highest)
Solution: C < A < B < D
Exercises 5.1
Order the following groups based on decreasing priority for E/Z naming purposes.

Answers to Chapter 5 Practice Questions
4. When a multiple bond is part of the group, the multiple bond is treated as if it was singly bonded to several of those atoms. Specifically:
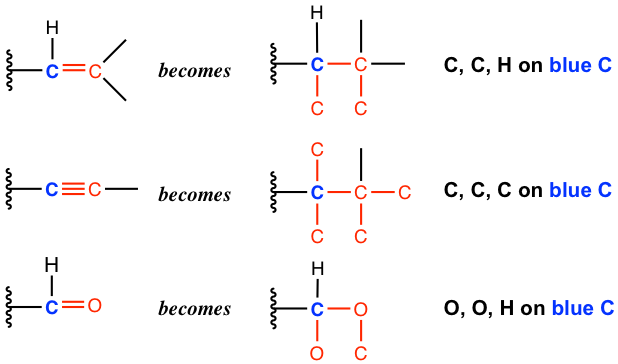
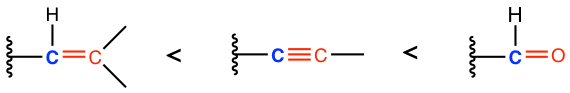
For these three groups that involve multiple bonds, they all start with the carbon atom (the carbon atom is highlighted in blue color), and we should compare the group of atoms that connected to the blue carbon by converting the multiple bonds to “multiple single bonds”, as shown above. So, if we compare the order of these three groups, it is:
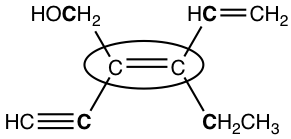
Examples: Assign E/Z of the circled double bond.
Thinking:


