Chapter 1: Basic Concepts in Chemical Bonding and Organic Molecules
1.2 Lewis Structure
A Lewis structure shows the bonding between atoms as short lines (some books use pairs of dots) and non-bonding valence electrons as dots.
1.2.1 Lewis Structure of Diatomic Molecules
To learn about Lewis structures, we will start with the Lewis symbol. The Lewis symbol is the chemical symbol of an element with valence electrons represented as dots. The Lewis symbols of some elements are shown here:
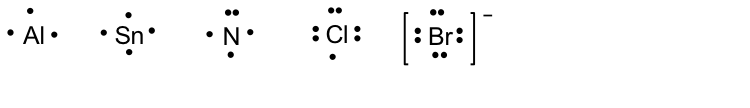
For simple diatomic molecules, combining the Lewis symbols of each element gives its Lewis structure.
H2 example: (H only needs two electrons; this is usually referred to as a duet.)
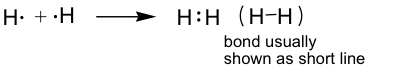
F2 example:
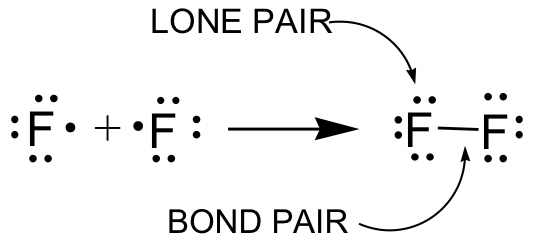
Terms used in Lewis structures (see example of F2):
- Bonding pair: The pair of valence electrons involved in a covalent bond. The covalent bonds are drawn as short lines in this book, and one covalent bond means one pair of bonding electrons, that is, 2 electrons. Single bonds and multiple bonds (double or triple bonds) may be involved.
- Lone pair: The pairs of valence electrons not involved in a covalent bond. Lone pair electrons can also be called non-bonding electron pairs.
Special note: Non-bonding electrons can also be unpaired (single) electrons. A species with one or more unpaired (single) electrons is called a radical (free radical). More examples of radicals with single electrons will be given in section 1.2.5 and Chapter 9.
HCl example:
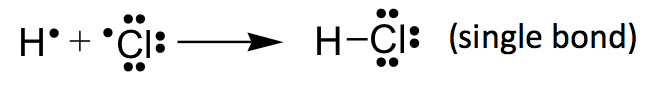
O2 example:
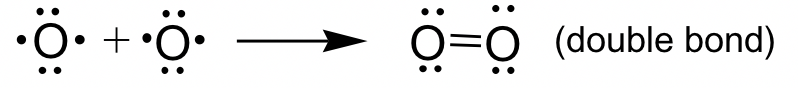
Exercises 1.3
Draw the Lewis structure of the N2 molecule.
Answers to Chapter 1 Practice Questions
1.2.2 Lewis Structures of Polyatomic Molecules or Ions
For more complicated polyatomic molecules and ions, the Lewis structures cannot be obtained by simply combining Lewis symbols. A specific procedure with certain steps has to be followed. It is very important that you use the following procedure to get the correct Lewis structures for polyatomic molecules and ions.
Lewis Structure Drawing Procedure for Polyatomic Molecules and Ions
1. Calculate the total number of valence electrons. For ions, make sure charges are properly included in the calculation. For example, for an NH4+ cation:
the total number of electrons = 5 (N atom) + 4×1 (four H atoms) -1 (minus the charge for cation) = 8 valence electrons
2. Write a plausible skeletal structure using the following steps:
a) Write atomic symbols for the central and terminal atoms.
- Hydrogen atoms are always terminal.
- Central atoms are generally those with the lowest EN.
- Carbon atoms are always central.
b) Connect the central atom with each of the terminal atoms by drawing a single bond.
3. For each single bond, subtract two electrons from the total number of valence electrons.
4. Using the remaining valence electrons, complete the octets of the terminal atoms first, then complete as many as possible for the central atoms.
5. If you have used up all the valence electrons to complete octets for all the atoms, you are done.
6. If not, then complete the octets of all central atoms by moving lone pairs from terminal atoms to form multiple bonds.
7. Calculate the Formal Charges on all atoms and label the non-zero formal charges in the structure:
Formal Charge on an atom = No. of valence electrons in free atoms–No. of lone pair electrons –½ (No. of bonding electrons)
Formula 1.1
Examples: Here we will take CO2 molecule as an example to explain the procedure step by step:
1. Total number of valence electrons: 4 (C atom) + 2×6 (2 O atoms) = 16
Always DOUBLE CHECK: In the correct Lewis structure, the total number of electrons involved (bonding plus non-bonding electrons) must be equal to this number, less or more are both incorrect!!
2. Write a plausible skeletal structure:
Carbon atoms are always central, so the skeletal structure is: O — C — O
3. Four electrons are used so far, and there are 16 – 4 = 12 electrons remained.
4. The remaining 12 electrons must be used to complete the octet for both terminal O atoms first, and no electrons left after that.
![]()
It is very important to keep in mind that the remaining electrons should be used to give the octet of terminal atoms first!
5. The central C atom does not get octet yet, we should do next step.
6. Moving one lone pair from each terminal O atom, the following structure is obtained.
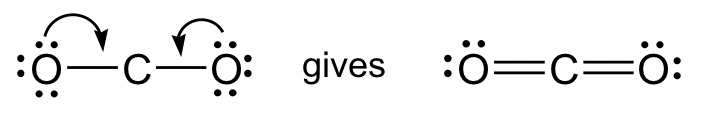 this is the complete Lewis structure of CO2.
this is the complete Lewis structure of CO2.
For Lewis structure purposes, the lone-pairs can only be moved from terminal atoms to the central atom to form multiple bonds, not the other way around.
7. Formal charges check: all atoms have formal charges equals to 0 in this structure.
FC (C) = 4 -½×(4×2) = 0
FC (O) = 6 -4-½×(2×2) = 0
Since the two oxygen atoms have the same bonding, one calculation is enough for both oxygen atoms.
1.2.3 Guidelines about Formal Charges in Lewis Structures
The purpose of formal charges is to compare the difference between the number of valence electrons in the free atom and the number of electrons the atom “owns” when it is bonded. The smaller the difference, the “happier” (more stable) the atom is. The atom owns all of the lone pair (non-bonding) electrons and half of the bonding (shared) electrons, which is why the formula is given in the way shown in Formula 1.1.
Formal charges can be used as guidelines to determine the plausibility of Lewis structures by comparing the stability of non-equivalent resonance structures, which is particularly important for organic species. The rules about formal charges are:
- The sum of the formal charges must equal to the total charge on the molecule or ion.
- Formal charges should be as small as possible (comparing the absolute value of formal charges for such purposes).
- “-” FC usually appears on the most electronegative atoms (with the stronger ability to pull the shared electrons; this atom is “winning” electrons in the sharing).
- “+” FC usually appears on least electronegative atoms (with the weaker ability to pull the shared electrons; this atom is “losing” electrons in the sharing).
- Structures having formal charges of the same sign on adjacent atoms is unlikely.
There is a derived way for calculating formal charges: since each bond contains 2 electrons, half of the bonding electrons simply equals the number of bonds. So, the formal charge can also be calculated based on the derived version of the formula:
Formal Charge on an atom = No. of valence electrons in free atoms–No. of lone pair electrons – No. of covalent bonds around the atom Formula 1.2
Double bonds count as 2 and triple bonds count as 3 in Formula 1.2. Both Formula 1.1 and 1.2 work for counting the formal charge; you can choose either one for your convenience. While almost all other textbooks show Formula 1.1 as the official way, Formula 1.2 is easier to use and can be regarded as the most practical one based on experience.
Exercises 1.4
Why is the following structure not the best way to show the Lewis structure of CO2 ?
![]()
Answers to Chapter 1 Practice Questions
1.2.4 Kekulé Structures vs Lewis Structures
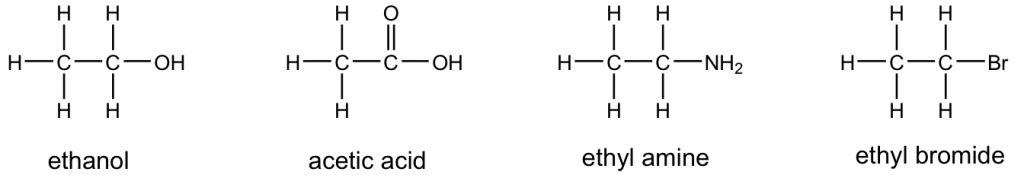
The complete Lewis structure always has to include all the bonding electrons and lone pair electrons. However, organic species are usually shown as Kekulé structures (further discussion will be given in Chapter 2) with all the lone pair electrons completely omitted (with exception to the lone pairs shown to highlight special properties). Therefore, when viewing Kekulé structures, it is helpful to keep in mind that atoms other than C and H should have a certain number of lone pairs. Examples of Kekulé structures of some compounds are given here:
1.2.5 Exceptions to the Octet Rule in Lewis Structure
So far, we have always been applying the octet rule in Lewis structures; however, there are some cases in which this rule does not apply. For example, H only needs 2 electrons. Here, we will see some other cases in which the octet rule is compromised.
- Odd number of electrons
If the total number of valence electrons is an odd number, the octet rule can not be applied to all atoms in the species. Examples could include NO (nitrogen monoxide or nitric oxide), NO2 (nitrogen dioxide) and alkyl radicals.
NO molecule: Although NO is a diatomic molecule, it is possible to draw the Lewis structure by following the procedure in section 1.2.2. Depending on which atom is given the octet first in Step 4, you may get two possible structures. By applying the formal charge guideline, we can decide that the first structure is the better choice with zero formal charges.
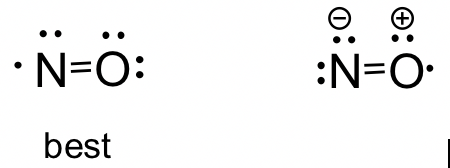
NO2 molecule: The Lewis structure of an NO2 molecule is shown below.
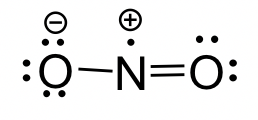
The above molecules all contain unpaired (single) electrons. The neutral species that contain an unpaired electron are called radicals (or free radicals). When the carbon atom of an alkyl group has an unpaired electron, the species is the alkyl radical.
Alkyl radicals: The simplest example of an alkyl radical is •CH3 with the total number of valence electrons as 7. The structure is:
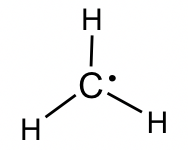
Further discussions about the properties and reactions of radicals will be included in Chapter 9.
- Incomplete Octet
An incomplete octet means the atom has less than 8 electrons involved. This could be because the total number of valence electrons is less than 8 or due to formal charge concerns.
BH3 molecule: The total number of valence electrons is 6, so the central boron atom does not get an octet.
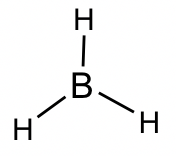
BF3 molecule: Even though all the atoms do have a chance to get octets in the structure of BF3, the actual structure of BF3 keeps the incomplete octet. Applying the FC guideline explains why the first structure is the better choice. Similar examples include BeF2 and AlCl3.
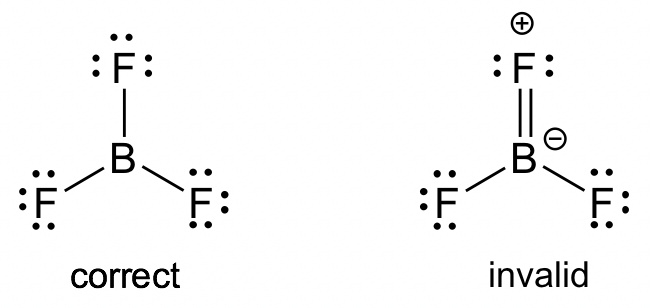
CH3+: This is another reactive intermediate in organic reactions (further discussions in Chapter 8). FC calculations indicate that the “+” charge lies on the C atom, so such a species is also called a carbocation. Carbon has an incomplete octet.
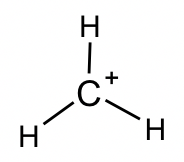
- Expanded Valence Shell
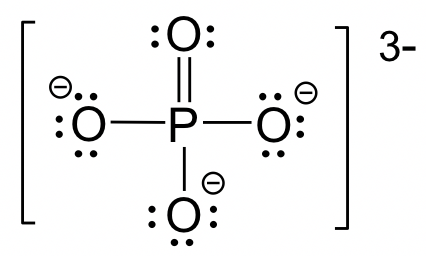
For elements in Period 3 or higher, they can have more than 8 electrons if it helps to lower the formal charges. Common examples involve species with P, S, Cl, etc. as central atoms. Sometimes multiple double bonds are necessary to minimize the formal charge of the central atom. The structure of the phosphate anion, PO43-, is given here as an example.
Key Takeaways
For elements in 2nd period, C, N, O, F and Ne, the maximum number of electrons involved in Lewis structure is eight!!!

