Chapter 2: Fundamentals of Organic Structures
2.4 IUPAC Naming of Organic Compounds with Functional Groups
With the ability to identify functional groups, next we will learn how to give IUPAC names to compounds containing several functional groups by following a set of rules.
IUPAC NOMENCLATURE of COMPOUNDS with FUNCTIONAL GROUPS
- Find the longest carbon chain containing the functional group with the highest priority (see Table 2.3). This chain determines the parent name of the compound.
- Change the ending of the parent alkane/alkene/alkyne to the suffix of the highest priority group, which gives the parent name of the compound (usually, drop the last letter “e” before adding the suffix, except for nitrile where the “e” is kept).
- Number the chain from the end closest to the highest functional group.
- The other groups are named as substituents by using the appropriate prefixes.
- Assign stereochemistry, E/Z or R/S, as necessary (details in Chapter 5).
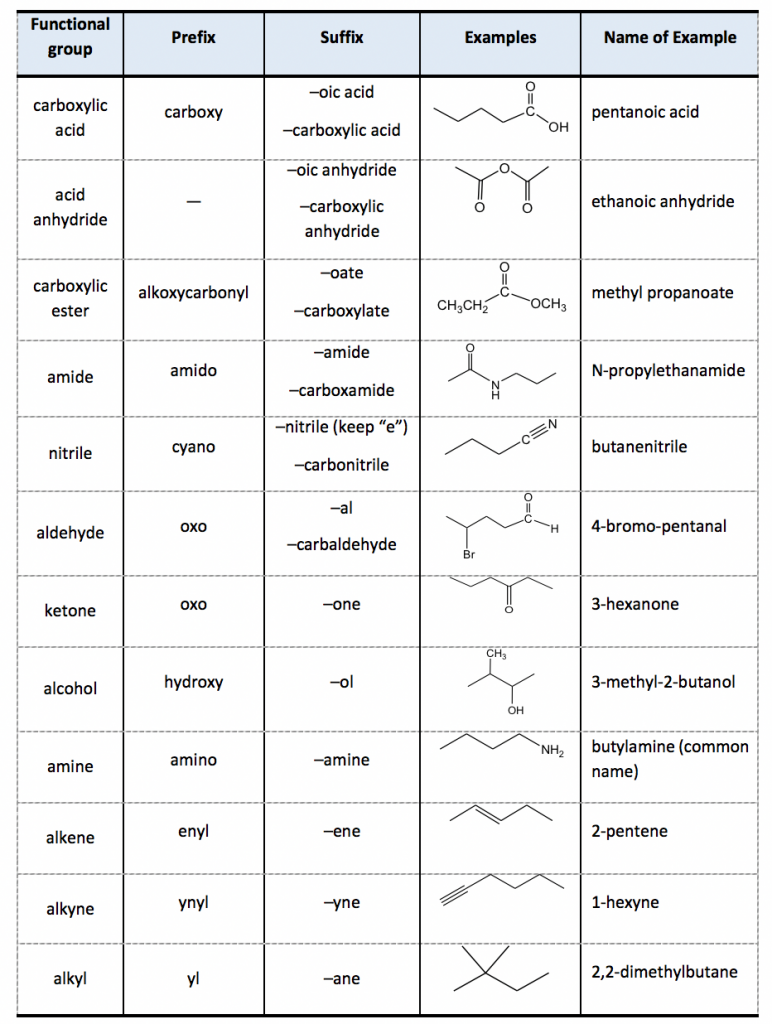
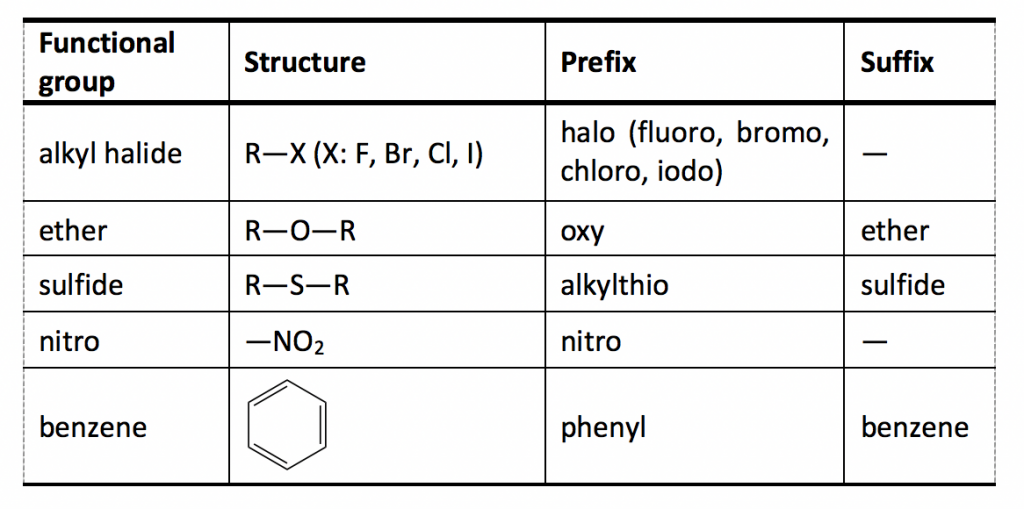
For naming purposes, the functional groups are assigned with priorities (Table 2.3). If the compound includes more than one functional group, the one with the highest priority is the “parent structure” and determines the “parent name”; the other groups will be regarded as “substituents”. The “suffix” is used to indicate the name of the parent structure, and the “prefix” is for the substituent. The order of the groups listed in Table 2.3 is based on the decreasing order of priority, where the carboxylic acid group is in the highest priority. The groups in the subordinate table (Table 2.4) have no difference in terms of priority, and they are usually listed in alphabetic order.
We will go through several examples for more details about the naming rules.
1.
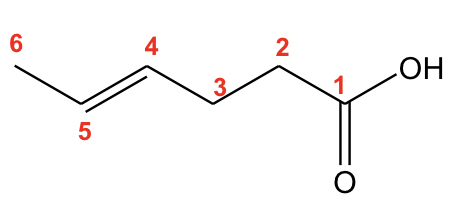
The parent structure is the 6-carbon carboxylic acid with a double bond, so the last name comes from “hexene”. To add the suffix, the last letter “e” will be dropped, so the parent name is “hexeneoicacid”. A number is necessary to indicate the position of the double bond, so the name is “4-hexenoic acid”. The carboxylic acid group is always in the #1 position, so it is NOT necessary to include that number for the position.
2.
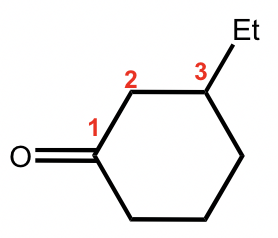
This is a ketone based on a cycloalkane, so the last name comes from “cyclohexane”. By adding the suffix, it becomes “cyclohexanone”, and the complete name is “3-ethylcyclohexanone”.
3.
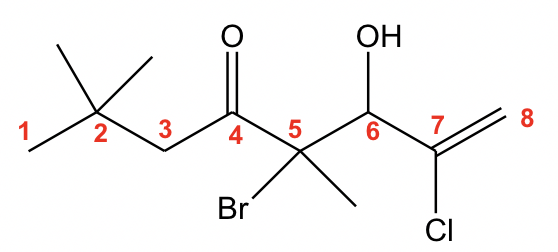
With the multiple groups involved, the ketone has the highest priority, so it decides the last name. The 8-carbon alkene chain with ketone should be named “octenone”. The numbers on the chain should start from the left side to ensure that ketone has the lowest number. When the OH group is regarded as a substituent, it is indicated by the prefix “hydroxy”. So the complete name is “5-bromo-7-chloro-6-hydroxy-2,2,5-trimethyl-7-octen-4-one”.
4.
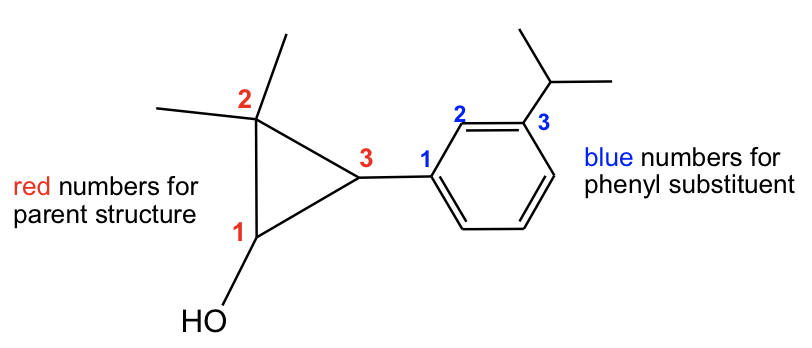
For this compound, it is not difficult to find the parent structure, which is a cyclic alcohol, so the last name is “cyclopropanol”. The naming of the substituent with the benzene ring is a bit more challenging. When benzene is a “substituent”, it is called “phenyl”; and since there is an isopropyl group on the “phenyl”, the whole substituent is called “3-isopropylphenyl”, and the complete name of the compound is “2,2-dimethyl-3-(3-isopropylphenyl)cyclopropanol”.
5.
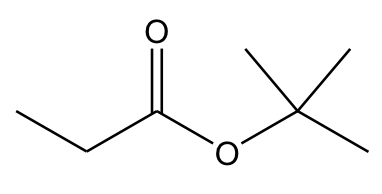
In ester, an OR group replaces the OH group of a carboxylic acid. When naming the ester, the name of the R in the OR group is stated first, followed by the name of the acid, with “oic acid” replaced by “oate”. As a net result, the R in the OR is regarded as the “substituent”, even though it is not. So, the complete name of the ester above is “tert-butyl propanoate”.
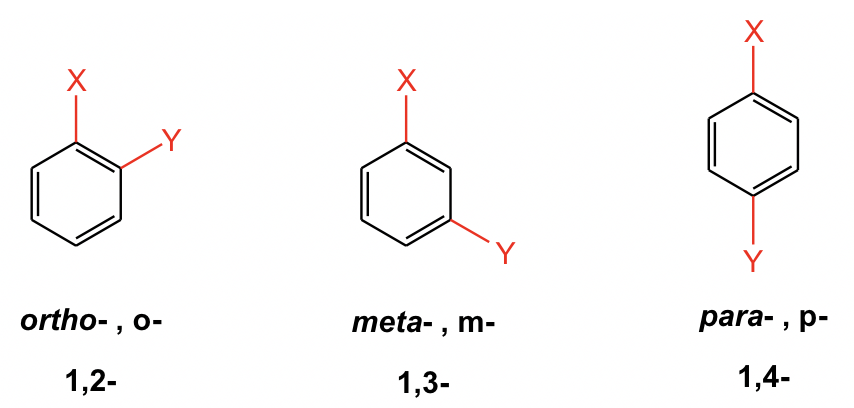
For substituted benzene, the benzene ring is regarded as the parent structure, and the positions and names of substituents are added to the front.
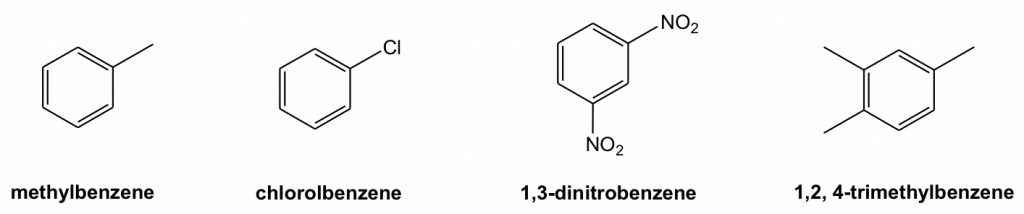
For di-substituted benzene, there is another unique way to indicate the relative position of the two substituents by using ortho-, meta- and para-. This o-, m-, p- system is the common naming system for benzene derivatives, however, they have been applied broadly in books and academic literature.
- ortho- (o-): 1,2- (next to each other in a benzene ring)
- meta- (m): 1,3- (separated by one carbon in a benzene ring)
- para- (p): 1,4- (across from each other in a benzene ring)
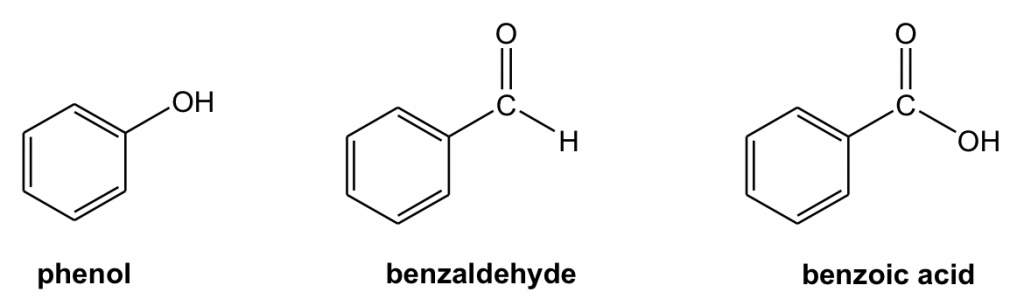
For the mono-substituted benzene derivatives: phenol, benzoic acid and benzaldehyde, their common names are adopted in the IUPAC system.
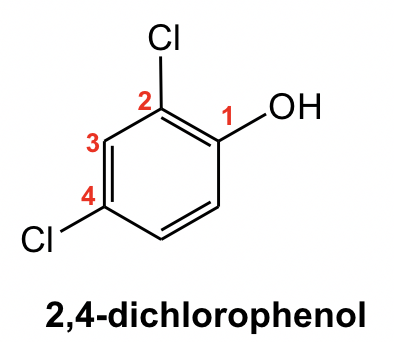
When other substituents are introduced into those benzene derivatives, the common name will be used as the parent name of the compound with the base functional group (OH for phenol, COOH for benzoic acid and CHO for benzaldehyde) given the #1 position. For example:
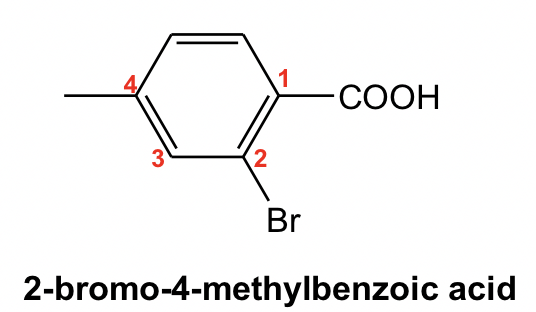
When benzene is connected with a carbon chain that has six or more carbons, the carbon chain should be regarded as the parent structure, and the benzene ring becomes the substituent and will be indicated with the prefix “phenyl”. An example is given here: