Chapter 2: Fundamentals of Organic Structures
2.1 Structures of Alkenes
2.1.1 Structures and Different Structure Formulas
Alkane is the simplest hydrocarbon with only C-C single bonds. The chain alkane fits the general formula of CnH2n+2 (n: positive integer), and the number of H atoms reaches the maximum level in chain alkanes. The names and structures of straight-chain alkanes up to ten carbons are listed in Table 2.1 below.
| Number of Carbons | Name |
Formula (CnH2n+2) |
Condensed Structure |
| 1 | methane | CH4 | CH4 |
| 2 | ethane | C2H6 | CH3CH3 |
| 3 | propane | C3H8 | CH3CH2CH3 |
| 4 | butane | C4H10 | CH3CH2CH2CH3 |
| 5 | pentane | C5H12 | CH3CH2CH2CH2CH3 |
| 6 | hexane | C6H14 | CH3CH2CH2CH2CH2CH3 |
| 7 | heptane | C7H16 | CH3CH2CH2CH2CH2CH2CH3 |
| 8 | octane | C8H18 | CH3CH2CH2CH2CH2CH2CH2CH3 |
| 9 | nonane | C9H20 | CH3CH2CH2CH2CH2CH2CH2CH2CH3 |
| 10 | decane | C10H22 | CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3 |
Table 2.1 Names and Structures of Straight-Chain Alkanes
The primary sources of alkanes are natural gas and petroleum. Natural gas contains mainly methane (70–90%) and some ethane. Petroleum refining separates crude oil into different fractions, and each fraction consists of alkanes of a similar number of carbons. Propane and butane are common fuels in propane gas burners and cigarette lighters. Alkanes with 5 to 8 carbons are the major components of gasoline, while diesel contains alkanes ranging from 9 to 16 carbons. As the number of carbons increases, the boiling point and viscosity of alkanes increase.
There are a variety of formats to show the structural formulas of organic compounds, and it is important to be able to recognize different formula drawings and use them correctly to represent the structures.
Kekulé Structure
We have had some discussions on Kekulé structures in section 1.2.4. They are similar to Lewis structures with all the bonding electrons shown in short lines and all the atoms included as element symbols. However, the lone pair electrons are left out in Kekulé structures, which is the major difference between Kekulé structures of organic compounds and Lewis structures.
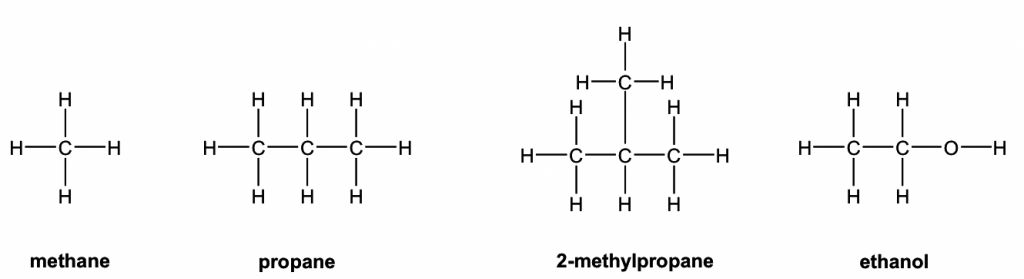
Condensed Structure Formula
In condensed structure formulas, the C-H bonds are omitted, and all the H atoms attached to a certain carbon (or other atoms) are usually shown as a group like CH3, CH2, NH2, and OH. The structures in Table 2.1 are shown as condensed structures. The C-C bond sometimes can be omitted as well (as for 2-methylpropane and 2-hexanol in the examples below). Usually, if the structure has a branch, the bonding between the parent structure to the branch needs to be shown with a short line. It is faster to draw a structure with a condensed structure formula, and it does not look as bulky as Kekulé structures.
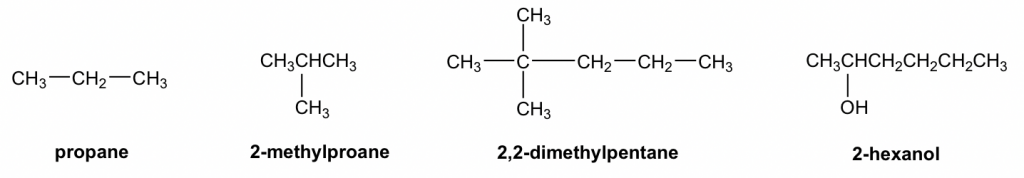
Short-Line Structure Formula
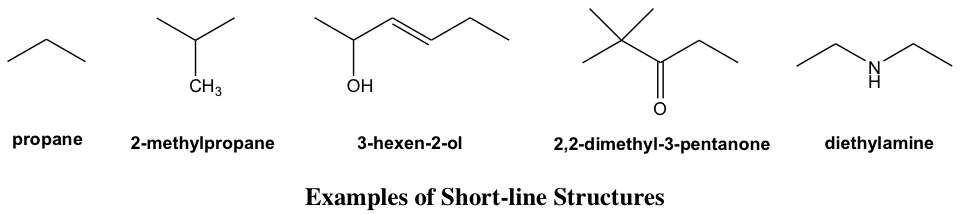
The structure drawing can be further simplified by a short-line structure (or “bond-line structure”, “skeletal formula” in other books) with most atoms omitted; this is also a very common type of structure formula used in organic chemistry because of its simplicity. To apply and interpret the short-line structures correctly, it is important to clearly understand the conventions of this type of drawing.
- Each short line represents a bond.
- The carbon chains are shown in a zig-zag way.
- No carbon atoms are shown (as an exception, it is optional to show the CH3 group at the end of the chain or as a branch); each bend in a line or terminus of a line represents a carbon atom unless another atom is shown explicitly.
- Hydrogen atoms bonded to carbons are not shown; hydrogen atoms bonded to other atoms are shown explicitly.
- Atoms other than C and H, for example, N, O, and Cl, need to be shown explicitly.
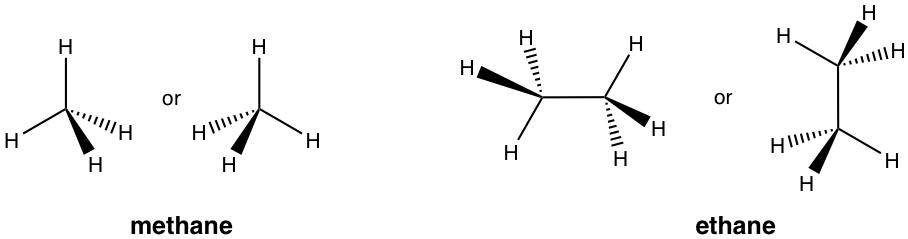
Perspective Formula of 3D Structure
When it is necessary to highlight the spatial arrangement of groups around a tetrahedral sp3 carbon for conformation (Chapter 4) or stereochemistry (Chapter 5) purposes, a perspective formula with solid and dashed wedges is used. Of the four bonds on a tetrahedral carbon, two bonds lie within the paper plane and are shown as ordinary lines, the solid wedge represents a bond that points out of the paper plane, and the dashed wedge represents a bond that points behind the paper plane.
2.1.2 Constitutional Isomers
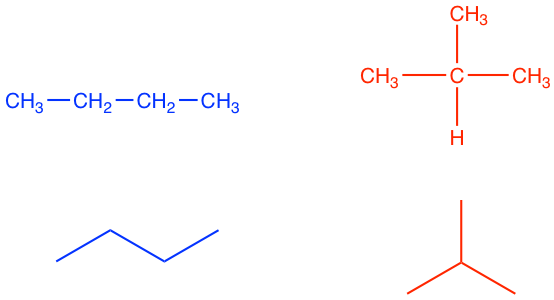
For methane, ethane and propane, there is only one type of carbon arrangement. As the number of carbon increases to 4, there are two ways for the carbon atoms to be connected: as a straight-chain (blue structure below) and as a branch on the chain (red structure below).
| Two Constitutional Isomers with Formula C4H10 | |
|
|
| Butane |
Isobutane (i-butane) “iso” means “isomeric” |
|
b.p. = 0°C |
b.p. = -12°C |
| density: 0.622 g/mL | density: 0.604 g/mL |
As we can see, these two different structures represent two different compounds, with different names and different physical properties; however, they both have the same formula of C4H10, and they are called constitutional (structural) isomers. Constitutional (structural) isomers are different compounds with the same molecular formula, but their atoms are arranged in a different order (i.e. the atoms are bonded in different ways).
Let’s see some more examples of constitutional isomers.
For alkanes with 5 carbons, there are a total of three constitutional isomers. The strategies for building constitutional isomers are given in the notes beside the structures.

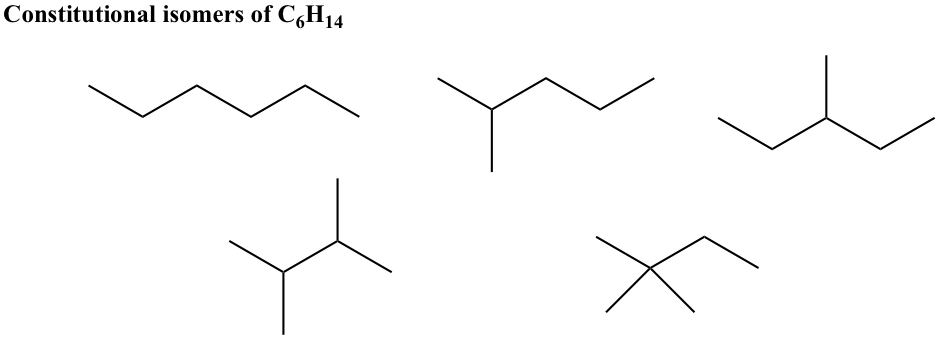
Exercises 2.1
Draw all the constitutional isomers with a formula of C7H16.
Answers to Chapter 2 Practice Questions
The constitutional isomers we have discussed so far have different lengths of carbon “backbones” and are also called skeletal constitutional isomers. The other possible situations include positional and functional constitutional isomers, which we will encounter later.
As the number of carbons increases, the number of constitutional isomers increases dramatically. For the example of alkanes with 20 carbons, that is C20H42, there are 366,319 constitutional isomers. While there is no simple formula that allows us to predict the total number of isomers for a certain number of carbons, the phenomena of constitutional isomers partially explain the high diversity of organic structures.
2.1.3 Recognition of 1°, 2°, 3°, 4°carbons
The carbon atoms in organic structures can be categorized as primary (1°), secondary (2°), tertiary (3°) and quaternary (4°), depending on how many other carbons it connects with. Specifically:
- Primary (1°) carbon: attached directly to only one other C atom
- Secondary (2°) carbon: attached directly to two other C atoms
- Tertiary (3°) carbon: attached directly to three other C atoms
- Quaternary (4°) carbon: attached to four other C atoms
The hydrogen atoms attached to 1°, 2° and 3° carbon are labeled as 1°, 2° and 3° hydrogen respectively.
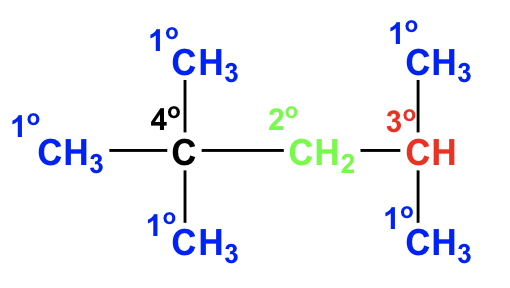
In one compound, carbons (or hydrogens) that belong to different categories show different structural and reactive properties. This concept has many more applications in later sections.

