Chapter 10: Alkenes and Alkynes
10.7 Oxidation Reactions of Alkenes
Alkenes undergo a number of reactions in which the C=C double bond is oxidized. For organic compounds, a conventional way to tell whether oxidation or reduction occurs is to check the number of C–O bonds or C–H bonds. An oxidation reaction increases the number of C–O bonds or decreases the number of C–H bonds. On the other side, a reduction reaction increases the number of C–H bonds or decreases the number of C–O bonds. The relative oxidation states of some common organic functional groups are listed here based on the trend.
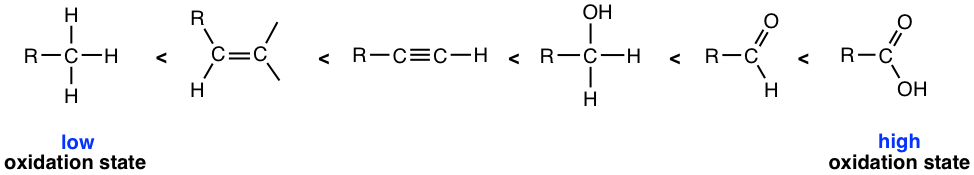
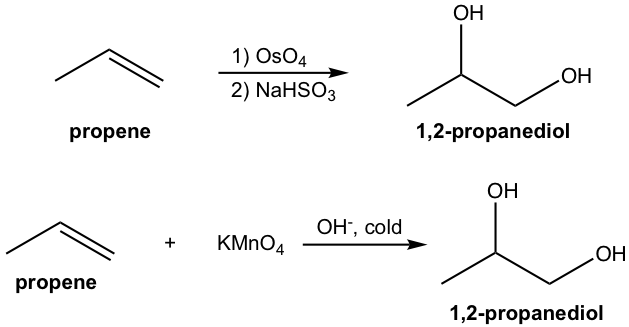
1,2-Dihydroxylation, the conversion of the C=C double bond to 1,2-diol, is an oxidative addition reaction of alkene. Osmium tetroxide (OsO4) is a widely used oxidizing agent for such purpose. Potassium permanganate can be used as well, though further oxidation is prone to occur to cleave the diol because it is a stronger oxidizing agent (10.7.2).
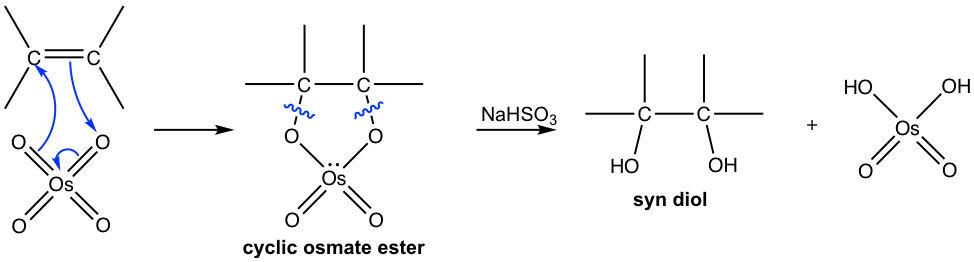
The traditional method of 1,2-dihydroxylation with osmium tetroxide is a two-step procedure. Osmium tetroxide first reacts with alkene to form a cyclic osmate ester intermediate, and this cyclic intermediate involves the syn addition of OsO4 to the double bond. The cleavage of the O—Os bond of the intermediate then takes place in the second step with the reducing agent NaHSO3 without modifying the stereochemistry of the C—O bond. The diol formed therefore has the syn stereochemistry property.
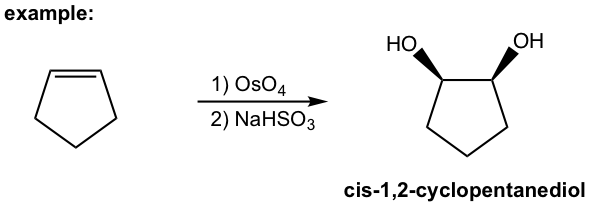
Catalytic OsO4 1,2-Dihydroxylation
The 1,2-dihydroxylation with osmium tetroxide is a reaction that is often used in labs for preparing diol from alkene effectively. However, this method has major drawbacks because osmium tetroxide is a highly toxic, volatile and expensive reagent. Improved methods have been developed that allow only a catalytical amount of OsO4 to be used in conjunction with a co-oxidant in a stoichiometric amount. N-methylmorpholine N-oxide (NMO) is one of the most commonly employed co-oxidants. In such conditions, osmium compounds are re-oxidized by NMO and can be reused to react with more alkenes, so only a small molar percentage of OsO4 is necessary in the reaction mixture. The reaction proceeds smoothly with syn diols produced in good yield.

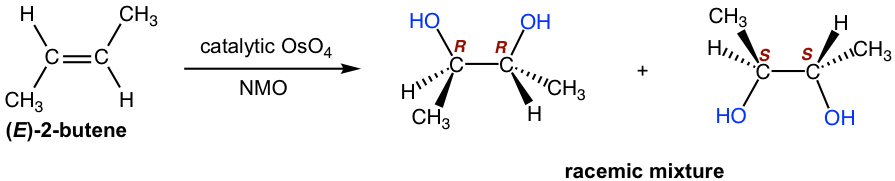
In terms of the stereochemistry of the product, although the syn addition could occur on either side of the alkene plane, it gives the same product, which is the meso compound. This can be identified by either looking for the plane of symmetry of the product or by assigning the absolute configuration on the chirality centers. Review the stereochemistry knowledge.
Examples
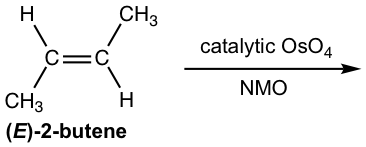
Solution:
The syn addition occurs on either side of the alkene plane, so both enantiomers are obtained with the same amount as a racemic mixture.
10.7.2 Oxidative Cleavage of Alkenes
Cleavage with Ozone
With a stronger oxidizing agent being applied, the C=C double bond of alkenes can be oxidatively cleaved, and the alkene molecule is cleaved into smaller molecules.
The most effective way to cleave alkene is to use ozone, O3, through a two-step process. Alkene first reacts with ozone at a very low temperature (-78°C) and then is treated with dimethyl sulfide (CH3)2S, (or Zn/CH3COOH) to give the cleavage products. The whole process is called ozonolysis.
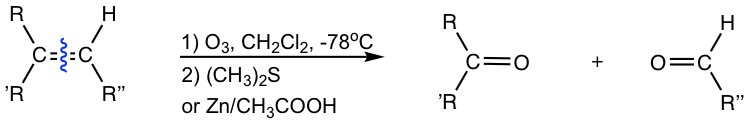
Ozonolysis results in the cleavage of the double bond, and each double bond carbon gets bonded to an oxygen atom with a new double bond. The products of ozonolysis are aldehyde(s) and/or ketone(s), and the exact structures of the products depend on the structure of the initial alkene:
- Disubstituted alkene carbons are oxidatively cleaved to ketone.
- Monosubstituted alkene carbons are oxidatively cleaved to aldehyde.
- Unsubstituted alkene carbons are oxidatively cleaved to formaldehyde (HCHO).
Examples
Show ozonolysis products of the following reactions:
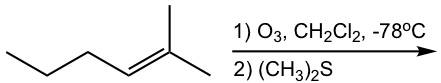
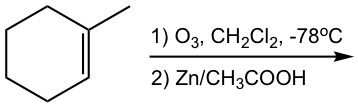
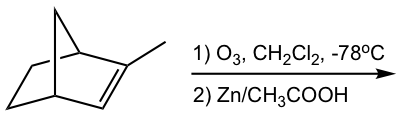
Hint: To figure out the structure of ozonolysis product(s), “cut” the double bond, then “add” a “=O” (double bonded oxygen) to each carbon.
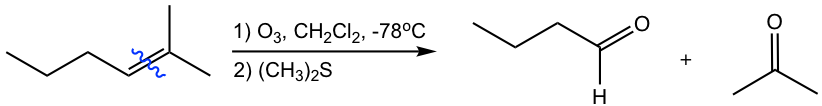
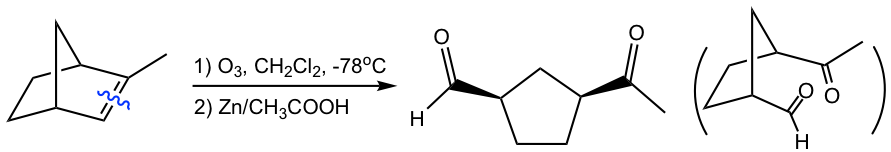
As shown in the above examples, the ozonolysis reaction is useful as a synthetic tool for certain aldehydes and ketones. Meanwhile, it is also a method for determining the position of double bonds in an alkene by working backward from the structure of the products.
Examples
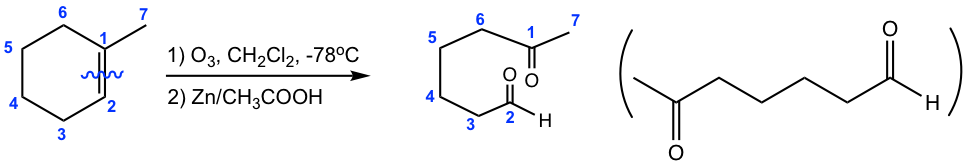
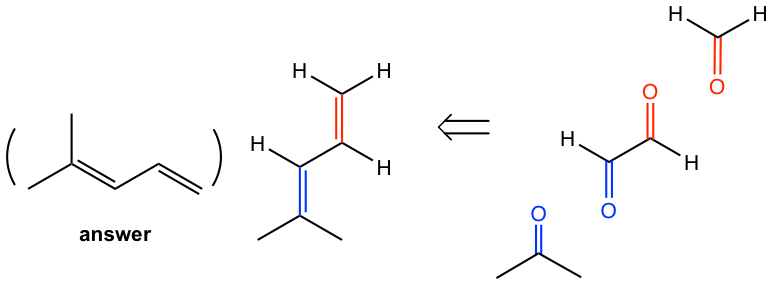
Determine the structure of the alkene:
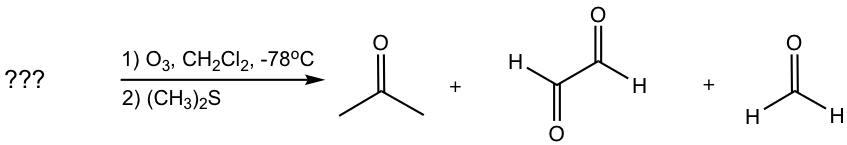
Approach: To determine the structure of the initial alkene, we can work backward by connecting two C=O bonds in the products together. The two C=O bonds are “connected” to give a C=C bond with all oxygen atoms “removed”. In this example, the two blue C=O bonds give the blue C=C bond, and the two red C=O bonds give the red C=C bond.
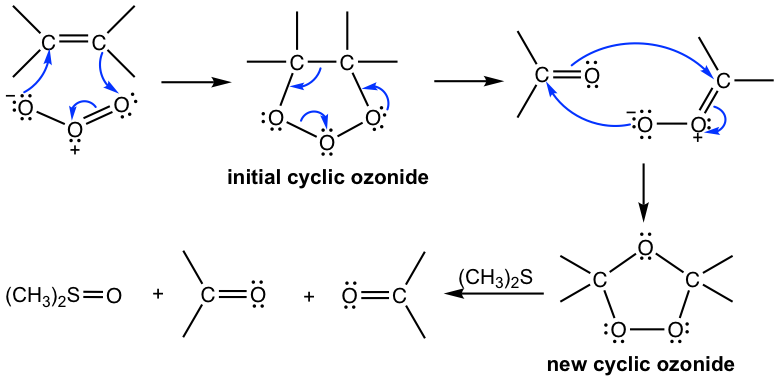
Mechanism for Ozonolysis
The hints mentioned earlier help us solve the problems with ozonolysis reactions, not the reaction mechanism. The mechanism of the ozonolysis reaction is complicated and involves the formation of an initial cyclic ozonide that decomposes into fragments, and the fragments recombine to form a new cyclic ozonide, which is reduced to give products.
Cleavage with Potassium Permanganate KMnO4
Potassium permanganate, KMnO4, is another oxidizing agent that cleaves the C=C double bond of an alkene. Under hot basic conditions, the oxidative cleavage products of alkenes could involve ketone, salt of carboxylic acid or carbon dioxide depending on the different substituent patterns on the alkene:
- Disubstituted alkene carbons are oxidatively cleaved to ketone.
- Monosubstituted alkene carbons are oxidatively cleaved to the carboxylic acid (in salt format).
- Unsubstituted alkene carbons are oxidatively cleaved to CO2 and H2O.
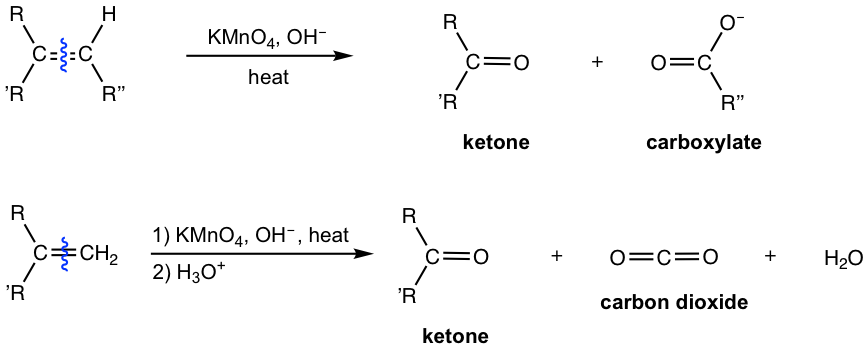
KMnO4 is a stronger oxidizing agent that further oxidizes the initial cleavage products, therefore aldehyde is further oxidized to carboxylic acid (in salt format under basic conditions). For terminal unsubstituted alkene carbons, the initial product is HCHO, which is then further oxidized to carboxylate CO32-in basic conditions. The acidification of CO32-produces H2CO3 which decomposes to CO2 and H2O. Because of over oxidation, KMnO4 is not a useful reagent for the synthesis of aldehyde/ketone from alkenes.

