Chapter 7: Nucleophilic Substitution Reactions
7.1 Nucleophilic Substitution Reactions Overview
Let’s start with a simple substitution reaction example:
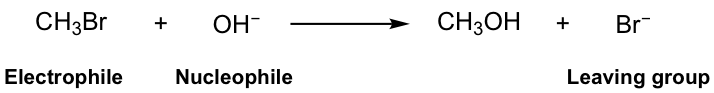
In this reaction, the Br in the reactant methylbromide (CH3Br) is replaced by the OH group, and methanol (CH3OH) is the major product, together with bromide Br-, the side product. It is easy to understand that this is a substitution reaction because Br is substituted by OH.
Further discussion on this simple reaction requires the introduction of some key terms that are critical for understanding why and how the reaction proceeds in this way. These terms are electrophile, nucleophile, and leaving group.
Electrophile
The reactant CH3Br is an alkyl halide. The C-X bond (X: F, Cl, and Br) in an alkyl halide is polar because halogen is more electronegative than carbon, and as a result carbon has a partial positive charge and halogen has a partial negative charge.
![]()
Because of the partial positive charge on carbon, the carbon atom in the C-X bond is electron-deficient, and it is going to seek an electron-rich reagent to connect with. Such an electron-deficient species is called an electrophile (phile is a Greek suffix meaning “love”, which indicates it is a species that loves electrons). Electron-deficient species are usually electrophiles. Other electrophile examples include positively charged ions and atoms with incomplete octets, for example, H+, CH3+, BH3, BeF2, and AlCl3.
For CH3Br in this reaction, it is the carbon atom that acts as the electrophile, and the carbon can be called an electrophilic carbon.
The hydroxide, OH–, is another reactant in the above reaction. It is shown clearly with the Lewis structure of OH– that the oxygen atom has three lone pair electrons and is negatively charged, so it is an electron-rich species with a high electron density.
![]()
An electron-rich species is called a nucleophile (“nucleo” comes from nucleus, which means positive charge), which is a reagent seeking positively charged or electron-poor species to react with. OH– is the nucleophile for the above reaction. Generally, any species with an electron pair available for sharing can be a nucleophile. A nucleophile can be either negatively charged (Nu:–), or neutral (Nu:), for example: OR–, H2O, ROH, NH3, RNH2, and RCOO– are all possible nucleophiles.
Based on the understanding of the concepts of electrophile and nucleophile, you have probably realized that a nucleophile can react with an electrophile! Yes, that is a very important and fundamental rule for organic reactions: when electron-rich nucleophiles meet with electron-deficient electrophiles, organic reactions can occur.
Leaving Group
To ensure the above substitution occurs, another critical factor is that the Br must leave together with the electron pairs in C-Br bonds, and the bromide, Br-, is called the leaving group. The leaving group (LG) leaves with the bonding pair of electrons and is replaced by the nucleophile in the substitution reaction. Without a proper leaving group, even a nucleophile is attracted to an electrophile, and the substitution reaction still cannot move forward. Leaving groups can be negatively charged or neutral, as we will see in detailed discussions later.
Applying the three key terms, the above substitution reaction can be summarized as: the nucleophile displaces the leaving group in a substrate, so such a reaction is called a nucleophilic substitution reaction. A nucleophilic substitution reaction can therefore be shown in a more general way:
![]()
Note: the nucleophile and leaving group are not necessarily negatively charged, as they could be neutral as mentioned earlier.
Kinetics of Nucleophilic Substitution Reaction
Kinetics is the study of the rate of a chemical reaction or how fast the reaction occurs. Reaction rate data helps shed light on the understanding of reaction mechanisms or the step-by-step electron transfer process. Kinetic studies on nucleophilic substitution reactions indicate that there are two different rate law expressions for such reactions. For the two reactions below, reaction 1 is in the second order, while reaction 2 is in the first order. The only reason for the different kinetic rates is that the reactions go through different reaction mechanisms.
![CH3Br + OH- = CH3OH + Br-, Reaction Rate = k times [CH3Br] times OH-](https://kpu.pressbooks.pub/app/uploads/sites/139/2020/11/Kinetics-rx1.png)
Reaction 1 is the substitution reaction we are already familiar with. It is a second-order reaction. That means the reaction rate depends on the concentration of both the substrate CH3Br and nucleophile OH–. If the concentration of CH3Br is doubled, the reaction rate gets doubled, and if the concentration of OH– is doubled, the reaction rate is doubled as well. When the concentration of both CH3Br and OH– are doubled, the reaction rate increases by a factor of four.
![(CH3)3CBr + H20 = (CH3)3COH + HBr, reaction rate = k times [(CH3)3Br]](https://kpu.pressbooks.pub/app/uploads/sites/139/2020/11/Kinetics-rx2.png)
Reaction 2 is another substitution reaction example. The substrate here is a tertiary bromide and the nucleophile is a neutral water molecule. As a first-order reaction, the reaction rate depends only on the concentration of the substrate (CH3)3CBr and has nothing to do with nucleophiles.
The two types of reactions correspond to two types of reaction mechanisms:
- A second-order reaction goes through the bimolecular reaction mechanism that is called an SN2 reaction, meaning Substitution, Nucleophilic and Bimolecular.
- A first-order reaction goes through the unimolecular reaction mechanism that is called an SN1 reaction, meaning Substitution, Nucleophilic and Unimolecular.
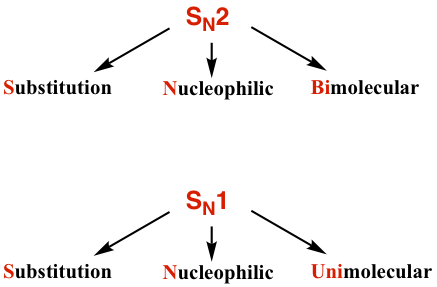
We will have detailed discussions on SN2 and SN1 mechanisms respectively, and then compare the similarities and differences between them.,.

