Chapter 4: Conformations of Alkanes and Cycloalkanes
4.2 Cycloalkanes and Their Relative Stabilities
While the open chain alkanes have conformational isomers because of bond rotation, will this apply to cycloalkanes as well? In this section, we will take a look at the properties of cycloalkanes first, then investigate how the different conformers of cycloalkanes contribute to the different stabilities.
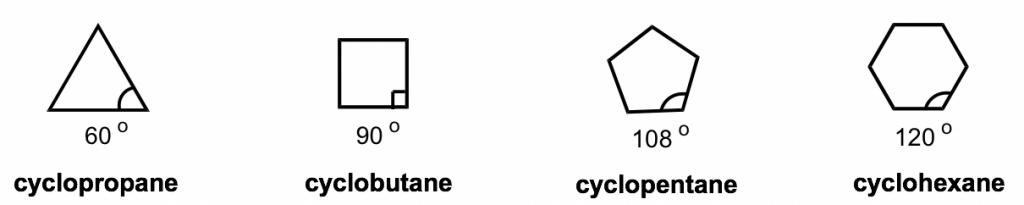
The short line structural formulas of cycloalkenes simply look like shapes such as a triangle, square, etc. The internal angles of the shapes can be calculated with geometry, as shown below.
An interesting fact about the cycloalkanes is that they have different relative stabilities, and the stability depends on the size of the ring. It has been observed that cyclic compounds found in nature are usually in 5- or 6-membered rings and 3- or 4-membered rings are rare.
To explain this stability difference, German chemist Adolf von Baeyer proposed the “Bayer Strain Theory”. By assuming all the rings are in a flat (or planar) shape, Bayer Theory suggests that the difference between the ideal bond angle (which is 109.5° for sp3 carbon) and the angle in the planer cycloalkane causes the strain, which is called angle strain. According to the Bayer Theory, cyclopentane would be the most stable because its bond angle, 108°, is closest to the ideal angle of 109.5°. Cyclopropane would be the least stable one since it has the largest angle deviation of 49.5° (60° vs 109.5°). It was also predicted that cyclohexane would be less stable than cyclopentane because of the larger angle deviation (10.5° deviation for cyclohexane vs 1.5° for cyclopentane), and as the number of sides in the cycloalkanes increases beyond six, the stability decreases.
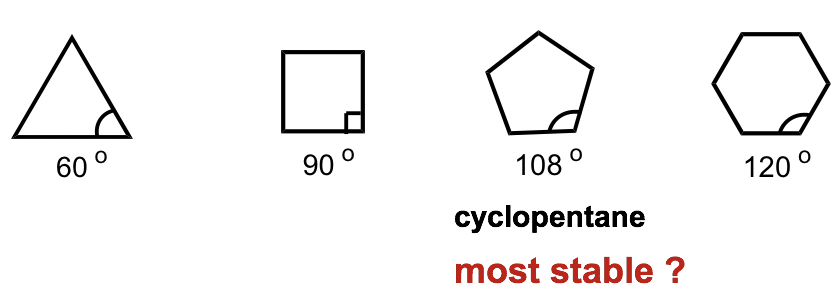
However, experimental results show a different trend. It turns out that cyclohexane is the most stable ring that is strain-free and is as stable as a chain alkane. Furthermore, cyclic compounds do not become less and less stable as the number of rings increases.
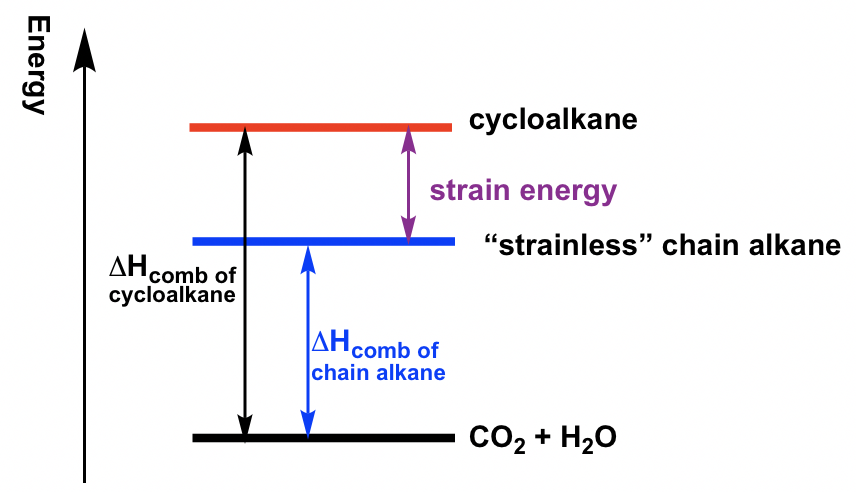
To measure the relative stability of cycloalkanes, the heat of combustion (ΔHcomb) for each cycloalkane was measured. The heat of combustion is the amount of heat released when the compounds burn completely with oxygen. The cycloalkanes will be in higher energy levels than corresponding chain alkanes because of strain energy. Therefore, when cycloalkane burns, more heat will be released, so the difference of ΔHcomb between cycloalkane vs the “strainless” chain alkane is just the amount of strain energy, as shown below. The larger the difference, the higher the strain energy of the cycloalkane. The strain energy for different cycloalkanes measured by this method is listed in Table 4.1.
| cyclopropane | cyclobutane | cyclopentane | cyclohexane | |
| Strain Energy (KJ/mol) | 114 | 110 | 25 | 0 |
Table 4.1 Strain Energies of Cycloalkanes
The major drawback of the Baeyer Theory is that we must assume that all the rings are flat. The highest stability of cyclohexane from experimental results indicates that the rings may not be in a planar shape. We will have a closer look at the actual shape and conformation of 3-, 4-, 5- and 6-membered cycloalkanes.
Cyclopropane
With three carbons for the ring, cyclopropane must be planar.
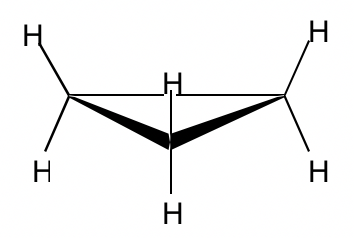
The bond angle in cyclopropane is 60°, derived significantly from the optimal angle of 109.5°, so it has very high angle strains. The sp3-sp3 orbitals can only overlap partially because of the angle deviation, so the overlapping is not as effective as it should be, and as a result, the C-C bond in cyclopropane is relatively weak.

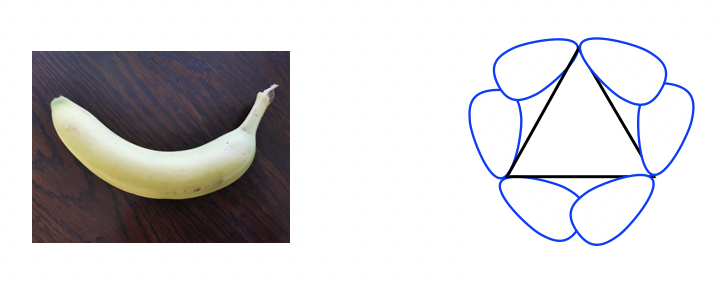
Because of the poor overlapping of sp3-sp3orbitals, the bonds formed in cyclopropane resemble the shape of a banana and are sometimes called banana bonds.
Other than the angle strains, all the adjacent C-H bonds are eclipsed in cyclopropane; therefore, the torsional strains are applied as well. Such strains can be “viewed” more clearly from the Newman projection of cyclopropane.
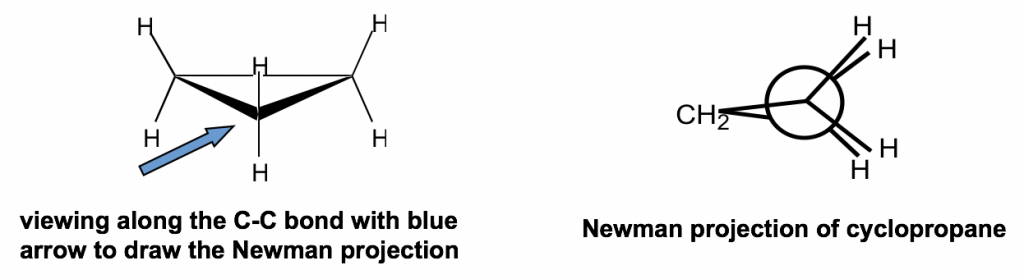
The Newman projection of cyclopropane might seem unusual at first glance. For cyclopropane, there are three carbons, so the CH2 group connects with both the front and rear carbons of the Newman projection.
Because of the high level of angle strains and torsional strains, 3-membered rings are unstable. They rarely exist in nature and undergo ring-opening reactions easily to release the strains.
Cyclobutane
Cyclobutane is not planar. The ring puckers (or folds) slightly due to the efforts of releasing some torsional strain. Meanwhile, cyclobutane still has a considerable number of angle strains as the internal angles become about 88° with the folded shape. Overall, cyclobutane is an unstable structure with a high level of strain.
Cyclopentane
Cyclopentane is also not planar, and the total level of strain is significantly lowered. It also puckers and adopts a bent conformation where one carbon atom sticks out of the plane of the others, which helps to release the torsional strain by allowing some hydrogen atoms to become almost staggered.
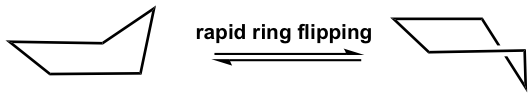
This bent shape of cyclopentane is also called the “envelope” conformation. The envelope conformation can undergo a process called “ring flipping” as a result of C-C bond rotation. Further discussion about ring flipping will be included in the section on cyclohexane.