Chapter 6: Structural Identification of Organic Compounds: IR and NMR Spectroscopy
6.1 Electromagnetic Radiation and Molecular Spectroscopy
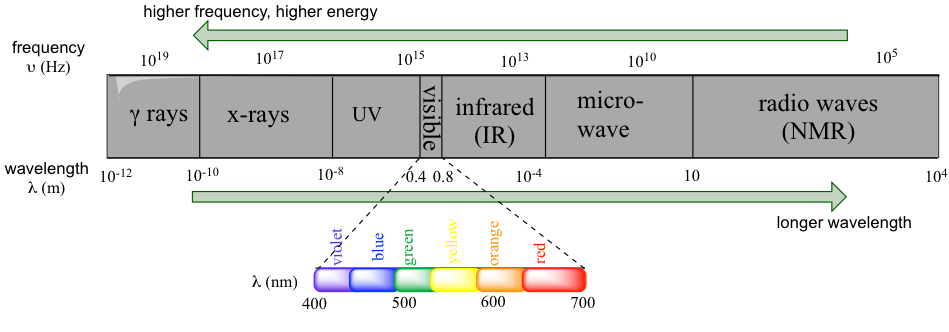
Electromagnetic radiation is radiation composed of oscillating electrical and magnetic fields. The whole electromagnetic spectrum covers radiation in a broad range from gamma rays (emitted by the nuclei of certain radioactive elements), X-rays (used for the medical examination of bones), to ultraviolet (UV) light (is responsible for sunburns and can also be used for disinfection purposes), microwaves, and radio-frequency waves (used for radio and television communication and for cell phone signals). Visible light, the radiation that is visible to our eyes and what we commonly refer to as “light”, accounts for only a very narrow band of the full electromagnetic spectrum.
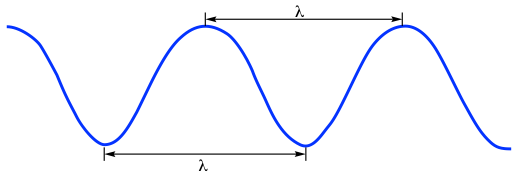
Electromagnetic radiation exhibits wave-like properties. As a general property of waves, the wavelength (λ, Greek ‘lambda‘) and frequency (ν, Greek ‘nu’, in a unit of Hz or s-1, 1Hz = 1s-1) of electromagnetic radiation fits the formula of:
c =λν Formula 6.1
where c is the speed, usually referred to as the “speed of light”, with the constant value of 2.998×108m/s in vacuum (the speed of light in air is a little bit slower than this constant but is usually regarded as the same). ). Because electromagnetic radiation travels at a constant speed, the wavelength (λ) and frequency (ν) are inversely proportional to each other: the longer waves have lower frequencies, and shorter waves have higher frequencies.
The energy of electromagnetic radiation can be calculated based on the formula:
E = hν= hc/λ Formula 6.2
where E is the energy of each photon in the unit of Joule (J) and h is the Planck’s constant with a value of 6.626×10-34J·s.
So, radiation with higher frequencies corresponds to higher energy. High energy radiation, such as gamma radiation and X-rays, is composed of very short waves – as short as 10 ~ 16m. Longer wavelengths are much less energetic and thus are less harmful to living things. Visible light waves are in the range of 400–700 nm (nanometer, 1 nm = 10-9m), while radio waves can be several hundred meters in length.
In a molecular spectroscopy experiment, the electromagnetic radiation of a specified range of wavelengths is allowed to pass through a sample containing a compound of interest. The sample molecules absorb energy from some of the wavelengths and as a result jump from a lower energy ‘ground state’ to a higher energy ‘excited state’. Other wavelengths are not absorbed by the sample molecule, and they pass through. A detector records which wavelengths were absorbed and how much was absorbed.
As we will see in this chapter, we can learn a lot about the structure of an organic molecule by quantifying how it absorbs (or does not absorb) different wavelengths in the electromagnetic spectrum. IR spectroscopy involves the absorption of radiations in the infrared region, and radio waves are applied in the NMR technique.

