Chapter 1: Basic Concepts in Chemical Bonding and Organic Molecules
1.6 Valence Bond Theory and Hybridization
1.6.1 Valence Bond Theory
We have discussed how covalent bonds are formed through the sharing of a pair of electrons; here we will apply the valence bond theory to explain in more detail how the sharing happens. The valence bond theory describes the covalent bond formed from the overlap of two half-filled atomic orbitals on different atoms.
Let’s start with the simple molecule H2. The atomic electron configuration of a hydrogen atom is 1s1, meaning there is one electron (which is also the valence electron) in the sphere-shaped 1s orbital.
When two hydrogen atoms are approaching each other, the two 1s orbitals overlap, allowing the two electrons (each H donates 1 electron) to pair up for the bonding with the overlapping orbitals. The shared pair of electrons are under the attraction of both hydrogen nuclei simultaneously, resulting in them acting as a “glue” that holds the two nuclei together.
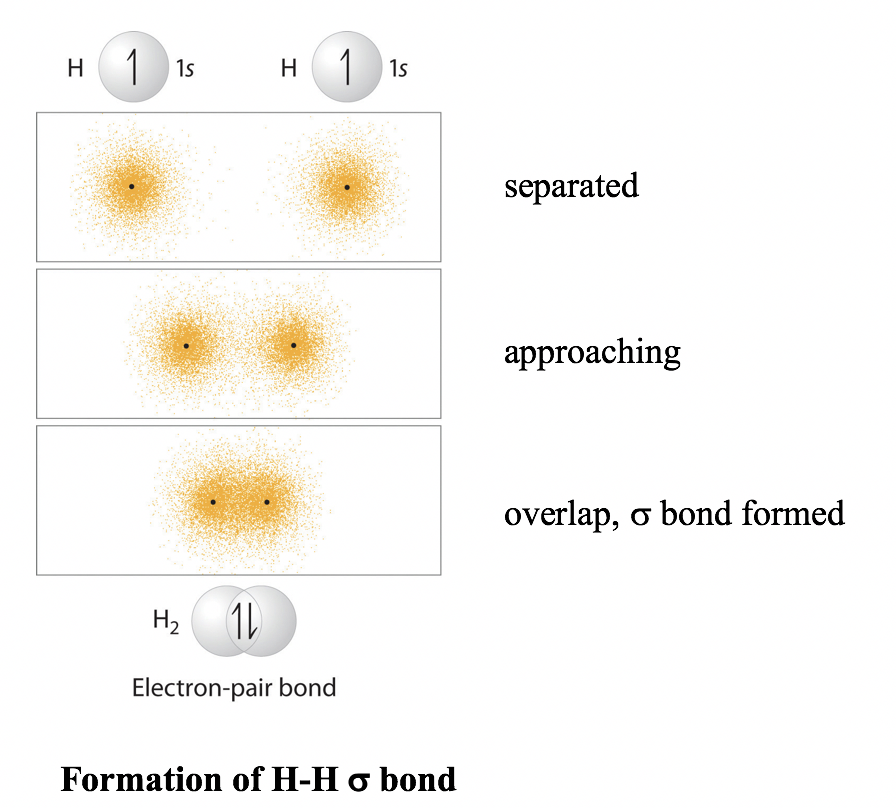
The overall energy changes of the system versus the distance between the two hydrogen nuclei can be summarized in the energy diagram below.
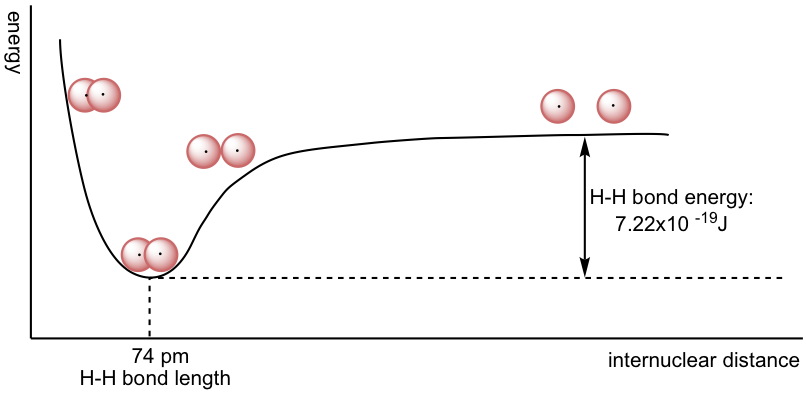
When the two atoms are separate, there is no overlap and no interaction. As they get closer, orbitals start to overlap, and there is attraction between the nucleus of one atom and the electron of the other atom, so the total energy of the system lowers. The energy lowers to its minimum level when the two atoms approach the optimal distance. The optimal distance is also defined as the bond length. H2 molecules have a bond length of 74 pm (often referred to as 0.74 Å, 1Å= 10-10m). The energy difference between the most stable state (lowest energy state with optimum distance) and the state in which the two atoms are completely separated is called the bond (dissociation) energy. The bond energy is 7.22×10-19 J for one H-H bond or 435 kJ/mol.
When the two atoms get closer than the optimal distance, the repulsion between the two nuclei becomes predominant, and the energy of the system becomes even higher.
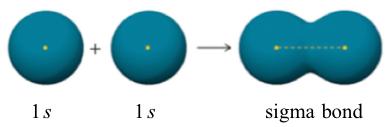
Another important character of the covalent bond in H2 is that the two 1s orbitals overlap in a way that is referred to as head-to-head. The bond formed by head-to-head overlap is called σ (sigma) bond. σ bonds are cylindrically symmetrical, meaning if a cross-sectional plane is taken of the bond at any point, it will form a circle.
The valence bond theory works well to explain the bonding in HF as well, with the 2p orbital of fluorine atom involved in the overlapping.
The fluorine atom has the valence electron configuration of 2s22p5 as shown in the orbital diagram.
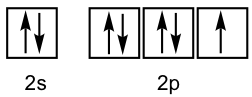
For the three 2p orbitals, two of them are filled, and the other one is half-filled with one single electron. The filled orbital cannot form bonds, so only the half-filled 2p is available for overlap. Therefore, the 1s orbital of the hydrogen atom overlaps head-to-head with the half-filled 2p orbital of the fluorine atom to form the H-F σ bond, as shown below.
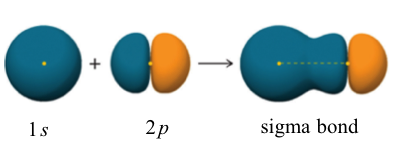
A σ bond can also be formed through the overlap of two p orbitals. The covalent bond in molecular fluorine, F2, is a σ bond formed by the overlap of two half-filled 2p orbitals, one from each fluorine atom as shown here.
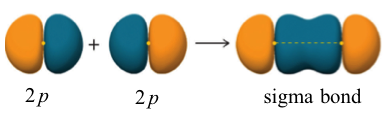
However, when the valence bond theory is applied to organic molecules, for instance CH4, it does not work. The valence electron configuration of a carbon atom is 2s22p2 as shown in the orbital diagram.
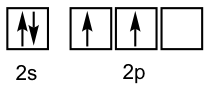
Based on the valence bond theory, with two half-filled orbitals available, the carbon atom should be able to form two bonds. However, carbon always has four bonds in any stable organic compound. To explain the bonding of carbon and other atoms that cannot fit into the simple valence bond theory, a new theory called orbital hybridization will be introduced as a supplement to the valence bond theory.
1.6.2 Hybridization and the Structure of CH4
Simply speaking, hybridization refers to the mathematical combination of several orbitals to generate a set of new hybrid orbitals.
In the hybridization for CH4, the 2s and three 2p orbitals are combined to give a new set of four identical orbitals that are called sp3 hybrid orbitals. The symbol sp3 here identifies the numbers and types of orbitals involved in the hybridization: one s and three p orbitals. For the hybridization process,
number of hybrid orbitals = the total number of atomic orbitals that are combined
It means that with a total of four orbitals combined, four new hybrid orbitals are generated, and they are all named sp3 hybrid orbitals. These new hybrid orbitals are all in the same energy level between those of 2s and 2p orbitals and are directed in a tetrahedral shape overall with the angle between any two orbitals as 109.5°. Each sp3 hybrid orbital has two lobes that are very different in size. The lobe with the larger size is in the positive phase and is responsible for bonding.
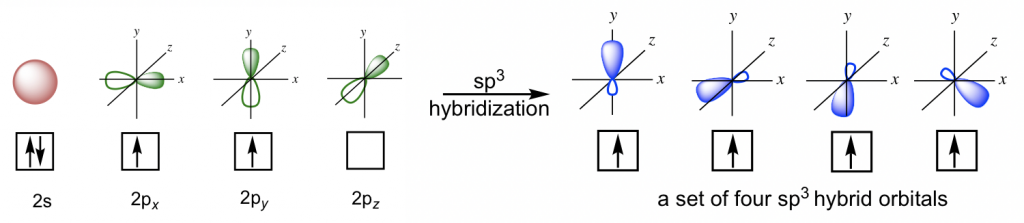

Since there are four sp3 hybrid orbitals available, each of the four valence electrons occupies one of them, so there are four half-filled sp3 orbitals in the carbon atom that are able to form four bonds. Therefore, the C-H bond of CH4 is formed by the overlapping between the 1s orbital in the hydrogen atom and the sp3 orbital in the carbon atom.
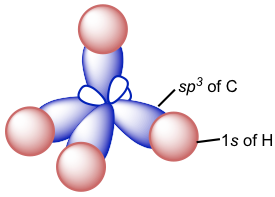
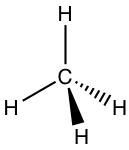
Because the arrangement of the four sp3 hybrid orbitals is in a tetrahedral shape, the shape of the CH4 molecule is also tetrahedral, which is consistent with the shape predicted by VSEPR. The tetrahedral shape of the sp3 carbon can usually be drawn using solid and dashed wedges. Out of the four bonds, the two bonds that lie within the paper plane are shown as ordinary lines, the solid wedge represents a bond that points out of the paper plane, and the dashed wedge represents a bond that points behind the paper plane. These perspective drawings that show the 3D tetrahedral shape are particularly important in the discussion of stereochemistry in Chapter 5.
1.6.3 Hybridization and VSEPR
Other than sp3 hybridization, there are also other types of hybridization that include sp, sp2, sp3d and sp3d2. Usually, the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and lone pairs). The total number of electron groups equals the total number of orbitals involved in the specific hybridization. For example, in a CH4 molecule, the central carbon atom has four bonding pairs, so the hybridization of carbon is sp3 (one s and three p orbitals, 1+3=4). If a central atom has a total of five electron groups (bonding pairs and lone pairs altogether), then the hybridization is sp3d (one s, three p and one d orbitals, 1+3+1=5).
This correlation may remind you of VSEPR. Hybridization and VSEPR are two separate concepts, but they can be correlated together via the number of electron groups in common. The following table (Table 1.4) is very useful in correlating the hybridization and VSEPR shape/bond angles around the central atom and the total number of electron groups together.
|
Hybridization on the central atom
|
Total number of electron pairs (BP and LP) around the central atom |
Geometry (Shape) of electron groups (electron pairs) |
|
sp |
2 |
linear |
| sp2 | 3 | trigonal planar |
| sp3 | 4 | tetrahedral |
| sp3d | 5 | trigonal bipyramidal |
| sp3d2 | 6 | octahedral |
Exercises 1.8
- What is the hybridization of the oxygen atom in H2O molecule?
- What is the hybridization of the xenon atom in XeF4 molecule, and what is the shape of the whole molecule?
Answers to Chapter 1 Practice Questions
1.6.4 The Hybridization and VSEPR in Organic Molecules
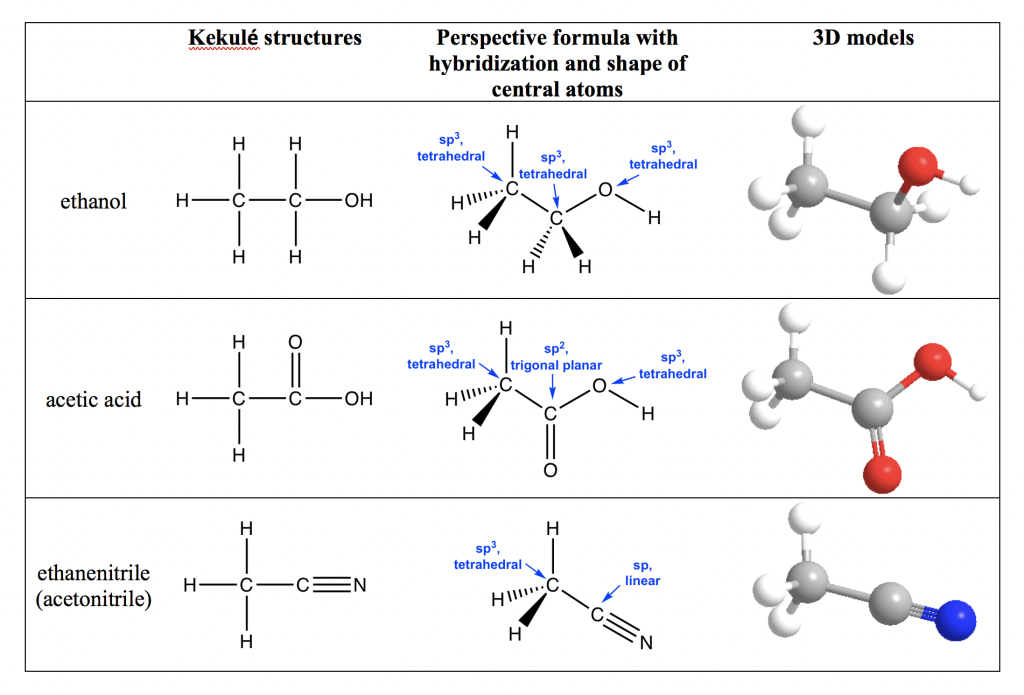
Organic molecules usually contain more than one central atom, so it is not practical to name the shape of the whole molecule; instead, we can talk about the shape/bond angle of each central atom individually. For such purposes, we must make sure to include the lone pairs that are usually left out in the organic structures (refer to section 1.2.4). The different structural formulas of ethanol, acetic acid and ethanenitrile molecules are shown in the table below. The 3D molecular model for each compound is shown as well to help you visualize the spatial arrangement. We can see that the hybridization and VSEPR shapes need to be separately indicated for each internal atom. Taking the oxygen atom in the OH group of ethanol as an example, since there are two pairs of lone pair electrons on the oxygen atom as well (these are omitted in the structures in the table), the oxygen has sp3 hybridization and is in a tetrahedral shape.
1.6.5 Multiple Bonds in Organic Structures
Ethene (C2H4)
We will take Ethene (C2H4) as an example for understanding the structure of a double bond.
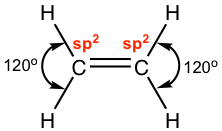
According to the structure formula of C2H4, there are three electron groups around each carbon. By referring to Table 1.3, it is determined that both carbons are in sp2 hybridization, with the trigonal planar shape and a 120° bond angle. What does the sp2 hybridization mean to the carbon atom in this compound? It means that only three orbitals are involved in the hybridization (one 2s and two of 2p orbitals) out of the total four, and there is one 2p orbital left out or not included in the hybridization, which is called the unhybridized 2p.
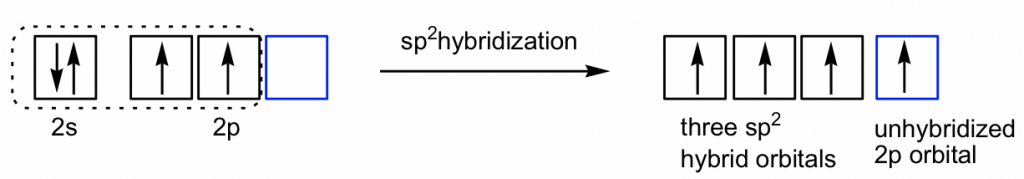
The three new sp2 hybrid orbitals and the unhybridized 2p are directed in the following arrangement: the three sp2 hybrid orbitals are in a trigonal planar shape, and the unhybridized 2p is in a position perpendicular to the plane. Each orbital has one single electron, so all the orbitals are half-filled and are available for bonding. Both carbon atoms have the same set of orbitals (three sp2 hybrid orbitals and one unhybridized 2p) as shown below.
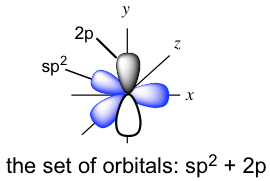
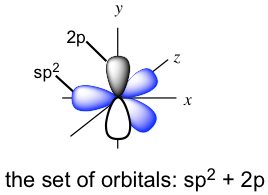
When the two carbons approach each other, the sp2 on the x-axis overlaps head-to-head to form the C-C σ sigma bond, and the “unhybridized” 2p overlaps side-by-side to form another new bond. The side-by-side orbital overlapping forms the π (pi) bond.
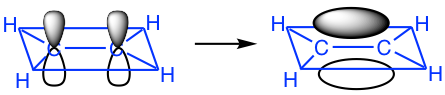
So now we understand that the C=C double bond contains two different bonds: the σ (sigma) bond from sp2–sp2 orbital overlapping and the π (pi) bond from 2p–2p overlapping. Because of the π bond, the overall shape of the whole C2H4 molecule is co-planar.
The other sp2 hybrid orbitals on each carbon atom overlap with 1s orbital of H atoms and give a total of four C-H σ (sigma) bonds.
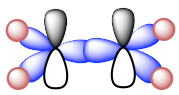
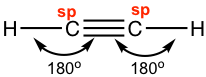
Ethyne C2H2 (common name is acetylene) has a C≡C triple bond. Generally, triple bonds involve one σ sigma bond and two π (pi) bonds. Both carbon atoms are in sp hybridization and in a linear shape. With sp hybridization, each carbon has two sp hybrid orbitals and two unhybridized 2p orbitals. Each carbon uses one sp hybrid orbital to overlap head-to-head and gives the C-C the σ sigma bond; meanwhile, the 2p orbitals overlap side-by-side to give two π bonds as shown in the diagram below. The other sp orbitals are used for overlapping with 1s of hydrogen atoms to form C-H σ bonds.
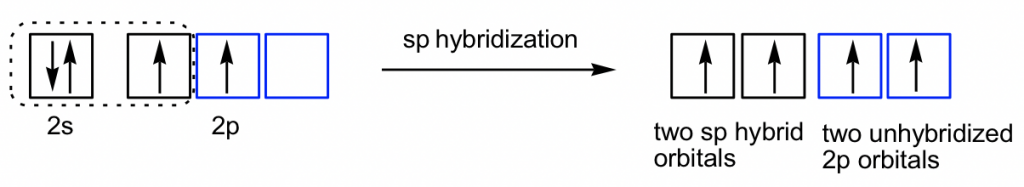
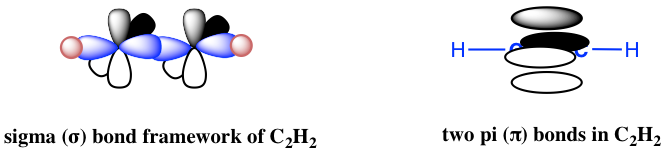
Image Descriptions
Table 1.5 image description: Ethanol’s CH3, CH2, and OH are all in a sp3 tetrahedral shape. Acetic acid’s CH3 and OH are in a sp3 tetrahedral shape, and CO is in a sp2 trigonal planar. Lastly, ethanenitrile’s (acetonitrile) CH3 is in a sp3 tetrahedral shape, and CN is in a sp linear shape. [Return to Table 1.5]

