Chapter 2: Fundamentals of Organic Structures
2.6 Intermolecular Force and Physical Properties of Organic Compounds
2.6.1 Intermolecular Forces
In organic chemistry, the understanding of the physical properties of organic compounds, for instance, boiling point (b.p.), molecular polarity and solubility, is very important, as it provides us with helpful information about how to deal with a substance in the proper way. Those physical properties are determined by the intermolecular forces involved. Intermolecular forces are the attractive forces between molecules that hold the molecules together; they are an electrical force in nature. We will focus on three types of intermolecular forces: dispersion forces, dipole-dipole forces and hydrogen bonds.
Dispersion Forces
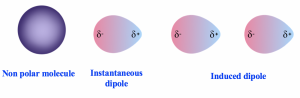
Dispersion forces (also called London forces) result from the instantaneous dipole and induced dipole of the molecules. For nonpolar molecules, the constant shifting and distortion of electron density leads to a weak, short-lived dipole at a given moment, which is called an instantaneous dipole. Such temporary dipoles also induce the electrons in a neighbouring molecule to be distorted, and this molecule develops a corresponding transient dipole of its own, which is the induced dipole. At the end, all nonpolar molecules are attracted together via the two types of temporary dipoles as shown in Fig. 2.6a. The dispersion force is weak in nature and is the weakest intermolecular force. However, since it applies to all types of molecules (it is the only intermolecular force for nonpolar molecules), dispersion forces are also the most fundamental intermolecular force.
The magnitude of dispersion forces depends on two factors:
- The relative polarizability of electrons – The simple understanding of polarizability is how easily the electrons get distorted. For larger atoms, there are more electrons in a larger space; therefore the electrons are more loosely held and more easily polarized, so the dispersion force is stronger. Generally, the larger the molar mass of the molecule, the stronger the dispersion force.
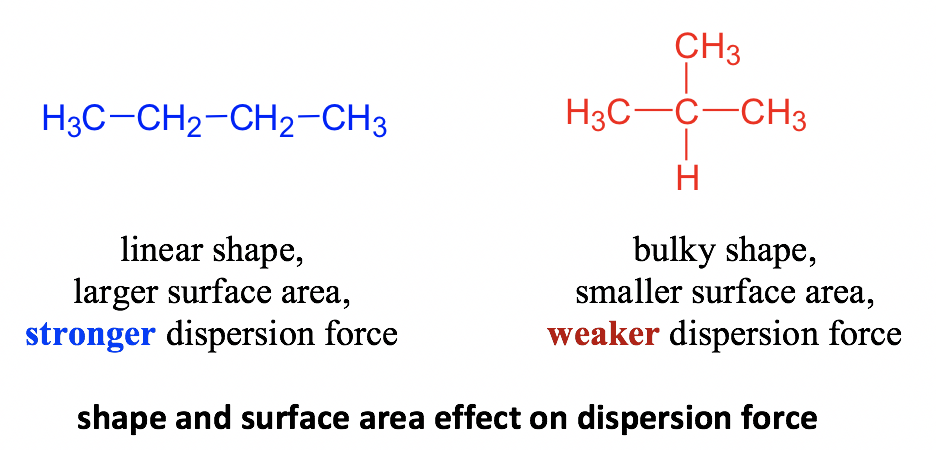
- The relative surface area of the molecule – Molecules with longer, flatter or cylindrical shapes have a greater surface area compared to the bulky, branched molecules, and therefore have a stronger dispersion force. Taking the two constitutional isomers of C4H10 (section 2.1.2), butane and isobutane as an example, the dispersion force of butane is stronger than that of isobutane.
Dipole-Dipole Force
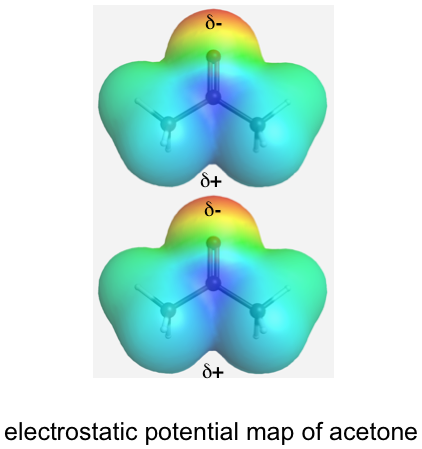
For polar molecules, molecules are attracted to each other because of a permanent dipole, this type of attractive force is called a dipole-dipole force. As shown below in the electrostatic potential map of acetone, one end of acetone has a partial negative charge (red), and the other end has a partial positive charge (blue). The dipole-dipole force is an attraction force between the positive end of one molecule and the negative end of the neighbouring molecule.
Hydrogen Bonds
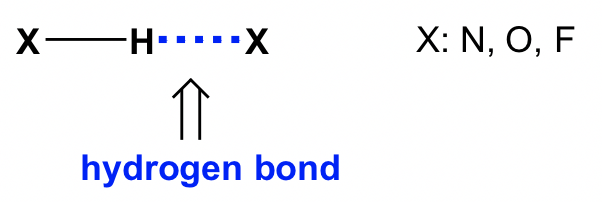
First of all, do not let the name mislead you! Although it is called a “bond”, a hydrogen bond is not a covalent bond, it is a type of intermolecular force. The hydrogen bond is the force between an H atom that is bonded to O, N or F (atoms with high EN) and the neighbouring electronegative atom. It can be shown in a general way as:
The most common example of hydrogen bonding is for water molecules. Water has two O-H bonds, and both are available as hydrogen bond donors for neighbouring molecules. This explains the extraordinarily high b.p. of water (100°C) considering the rather small molar mass of 18.0 g/mol. As a comparison, a methane molecule CH4 with a similar size has a b.p. of -167.7°C.
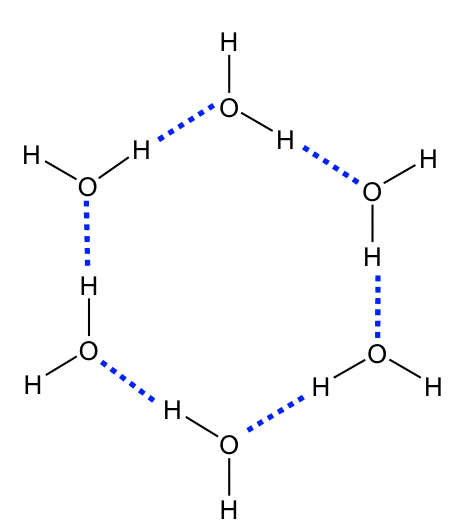
For organic compounds, hydrogen bonds play important roles in determining the properties of compounds with OH or NH bonds, for example, alcohol (R-OH), carboxylic acid (R-COOH), amine (R-NH2) and amide RCONH2.
The three major types of intermolecular forces are summarized and compared in Table 2.6.
| Type of Force |
Applied to |
Strength |
| Dispersion Forces | All molecules | 0.1 – 5 kJ/mol |
| Dipole-dipole Forces | Polar molecules | 5 – 20 kJ/mol |
| Hydrogen Bonding | Polar molecules with
N – H, O – H or F – H bond |
5 – 50 kJ/mol |
Table 2.6 Summary of the Three Major Intermolecular Forces
Polar vs Non-Polar molecules
As indicated in Table 2.6, the nature of molecular polarity determines the types of force(s) applied to a certain substance. So here we will have discussions about how to tell whether a molecule is polar or non-polar.
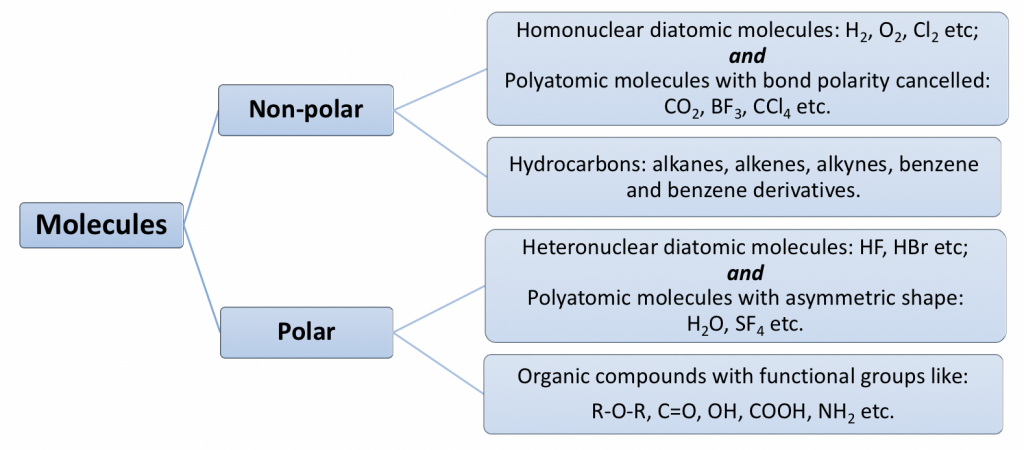
The polarity of the compound can be determined by its formula and shape.
For diatomic molecules, the molecular polarity is the same as the bonding polarity. That means all homonuclear molecules, like H2, N2, O2, and F2, are non-polar because of their non-polar bond, while all heteronuclear molecules, like HF and HCl, are polar.
For polyatomic molecules, the molecular polarity depends on the shape (refer to VSEPR in Section 1.5) of the molecule as well. Let’s see some examples of H2O and CO2.
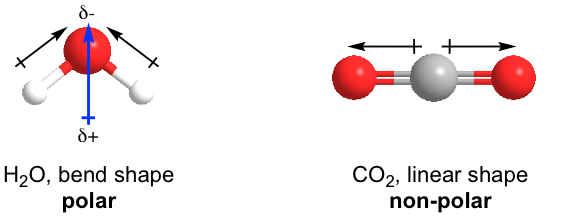
Both H2O and CO2 have two polar bonds. H2O is in a bent shape, so the bond polarities of the two O-H bonds add up to give the molecular polarity of the whole molecule (shown above); therefore, H2O is a polar molecule. On the other hand, the shape of CO2 is linear, and the bond polarities of the two C=O bonds cancel out, so the whole CO2 molecule is non-polar.
There are other examples of non-polar molecules where the bond polarity cancels out, such as BF3, CCl4, PCl5, and XeO4.
For organic compounds, the hydrocarbons (CxHy) are always non-polar. This is mainly because of the small EN difference between carbon atoms and hydrogen atoms, making C-H bonds technically non-polar bonds.
For other organic compounds that contain functional groups with heteroatoms, like R-O-R, C=O, OH, and NH, they are all polar molecules.
The diagram here (Fig. 2.6g) provides a summary of all the discussions about molecular polarities.
Other than the three types of intermolecular forces, another interaction is very important for understanding the physical property of a compound, which is the ion-dipole force.
Ion-Dipole Force
The ion-dipole force is not categorized as an intermolecular force, as it is an important non-covalent force that is responsible for the interaction between ions and other polar substances. A simple example is the dissolving of an ionic solid, or salt, in water. When table salt (NaCl) is dissolved in water, the interactions between the ions and water molecules are strong enough to overcome the ionic bond that holds the ions in the crystal lattice. As a result, the cations and anions are separated completely, and each ion is surrounded by a cluster of water molecules. This is called a solvation process. The solvation occurs through the strong ion-dipole force. Many salts, or ionic compounds, are soluble in water because of such interactions.
2.6.2 Physical Properties and Intermolecular Forces
The comprehension of intermolecular forces helps us understand and explain the physical properties of substances since intermolecular forces account for physical properties such as phases, boiling points, melting points, and viscosities. For organic chemistry purposes, we will focus on b.p. and solubility.
Boiling point (b.p):
The b.p. trend of different substances directly correlates with the total intermolecular forces. Generally speaking, the stronger the overall intermolecular force applied to a certain substance, the higher the b.p. of the substance. The b.p. is the temperature at which the liquid phase of the substance vaporizes to become a gas. To vaporize a liquid, the intermolecular forces that hold the molecules together must be overcome. The stronger the forces, the more energy that is needed to overcome the forces, and a higher temperature is required, thus leading to a higher b.p.
Example:
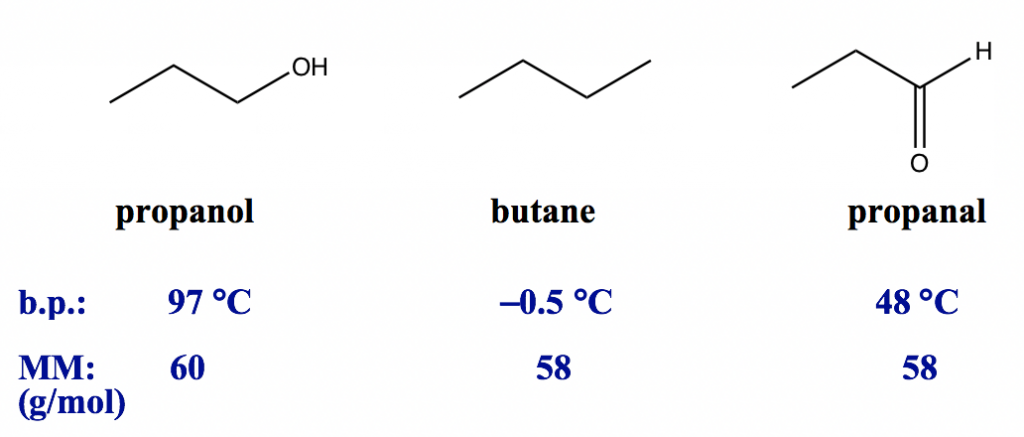 All three compounds here have similar molar masses, so the dispersion forces are at a similar level. However, the three compounds have different molecular polarities. Butane is a non-polar substance that only has dispersion forces, propanal is a polar molecule with both dispersion forces and dipole-dipole forces, and propanol is a polar molecule with an OH bond, so all three types of forces apply. Therefore, the overall amount of intermolecular force is strongest for propanol and weakest for butane, which is in the same order as their boiling points.
All three compounds here have similar molar masses, so the dispersion forces are at a similar level. However, the three compounds have different molecular polarities. Butane is a non-polar substance that only has dispersion forces, propanal is a polar molecule with both dispersion forces and dipole-dipole forces, and propanol is a polar molecule with an OH bond, so all three types of forces apply. Therefore, the overall amount of intermolecular force is strongest for propanol and weakest for butane, which is in the same order as their boiling points.
Solubility:
A general rule for solubility is summarized by the expression “like dissolves like”. This means that one substance can dissolve in another with a similar polarity and, as a result, with similar intermolecular forces. More specifically:
- Nonpolar substances are usually soluble in nonpolar solvents.
- Polar and ionic substances are usually soluble in polar solvents.
- Polar and nonpolar substances are insoluble to each other.
Determining the polarity of a substance has already been summarized in an earlier part of this section (Fig. 2.6g). Water, methanol and ethanol are examples of very polar solvents that can form hydrogen bonds. Ether, ketone, halide and esters are polar solvents as well, but they are not as polar as water or methanol. Non-polar solvents include hydrocarbons like hexane, benzene, and toluene.
For large organic molecules, however, it may not be so easy to simply call them polar or non-polar because part of a compound may be polar while another part may be nonpolar. Such polar or nonpolar parts of a compound are often described as hydrophilic or hydrophobic.
- Hydrophilic (hydro, water; philic: loving or seeking) means it likes water, or is soluble in water.
- Hydrophobic (hydro, water; phobic: fearing or avoiding) meaning it does not like water, or is insoluble in water.
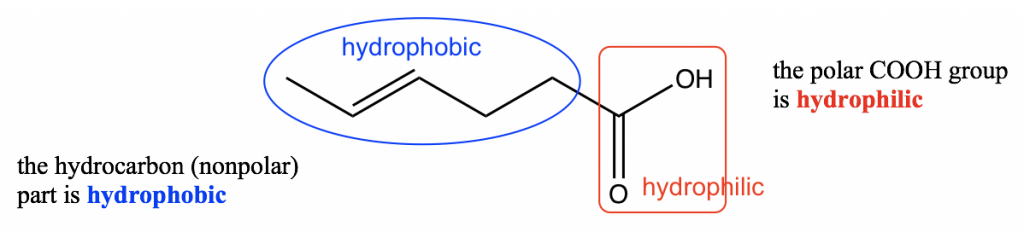
The hydrocarbon part of an organic compound is hydrophobic because it is nonpolar and therefore does not dissolve in polar water. The functional group of OH, COOH, NH2, etc. is polar and is therefore hydrophilic. With both hydrophobic and hydrophilic parts present in an organic compound, the overall polarity depends on whichever part is the major one. If the carbon chain is short (1~3 carbons), the hydrophilic effect of the polar group is the major one, so the whole compound is soluble in water; with carbon chains of 4~5 carbons, the hydrophobic effect begins to overcome the hydrophilic effect, and water solubility is lost.
The solubility differences of different alcohols demonstrate this trend clearly; as the length of the carbon chain increases, the solubility of alcohol in water decreases dramatically (Table 2.7):
| Alcohol |
Solubility in water (g/100mL) |
| methanol, ethanol, propanol
(CH3OH, CH3CH2OH, CH3CH2CH2OH) |
miscible (dissolve in all proportions) |
| 1-butanol (CH3CH2CH2CH2OH) |
9 |
| 1-pentanol (CH3CH2CH2CH2CH2OH) |
2.7 |
| 1-octanol (CH3CH2CH2CH2CH2CH2CH2CH2OH) |
0.06 |
Table 2.7 Solubility of different alcohols in water
For organic compounds that are water-insoluble, they can sometimes be converted to the “salt derivative” via a proper reaction and thus can become water-soluble. This method is commonly used in labs for the separation of organic compounds.
Example:
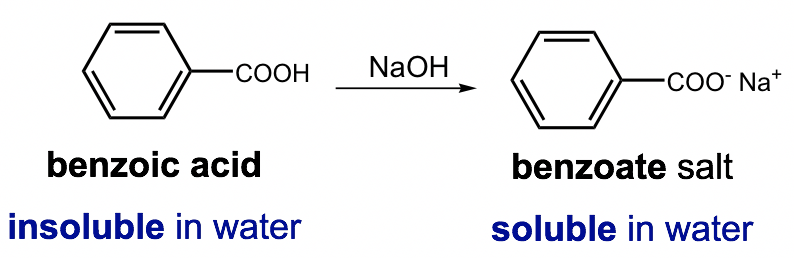
Applying acid-base reactions is the most common way of achieving such purposes. As shown in the above example, by adding a strong base to the benzoic acid, an acid-base reaction occurs and benzoic acid is converted to its salt, sodium benzoate, which is water soluble (because of the ion-dipole force, as we learned earlier). The benzoic acid can therefore be brought into the water (aqueous) phase and separated from other organic compounds that do not have similar properties.

