Chapter 10: Alkenes and Alkynes
10.3 Reactions of Alkenes: Addition of Water (or Alcohol) to Alkenes
Addition of Water to Alkenes (Hydration of Alkenes)
An alkene does not react with pure water since water is not acidic enough to allow the hydrogen to act as an electrophile to start a reaction. However, with the presence of a small amount of an acid, the reaction does occur with a water molecule added to the double bond of alkene, and the product is an alcohol. This is the acid-catalyzed addition reaction of water to alkene (also called hydration), and this reaction has great utility in the large-scale industrial production of certain low-molecular-weight alcohols.
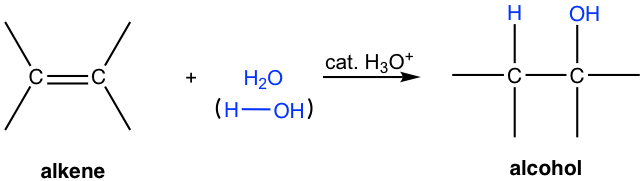
The acid most commonly applied to catalyze this reaction is the dilute aqueous solution of sulfuric acid (H2SO4). Sulfuric acid dissociates completely in an aqueous solution and the hydronium ion (H3O+) generated participates in the reaction. a strong organic acid, tosyl acid (TsOH), is sometimes used as well.
The mechanism for the acid-catalyzed hydration of alkene is essentially the same as the mechanism for the addition of hydrogen halide, HX, to alkenes, and the reaction therefore also follows Markovnikov’s rule in terms of regioselectivity. The hydration of 1-methylcyclohexene and the reaction mechanism is shown below.
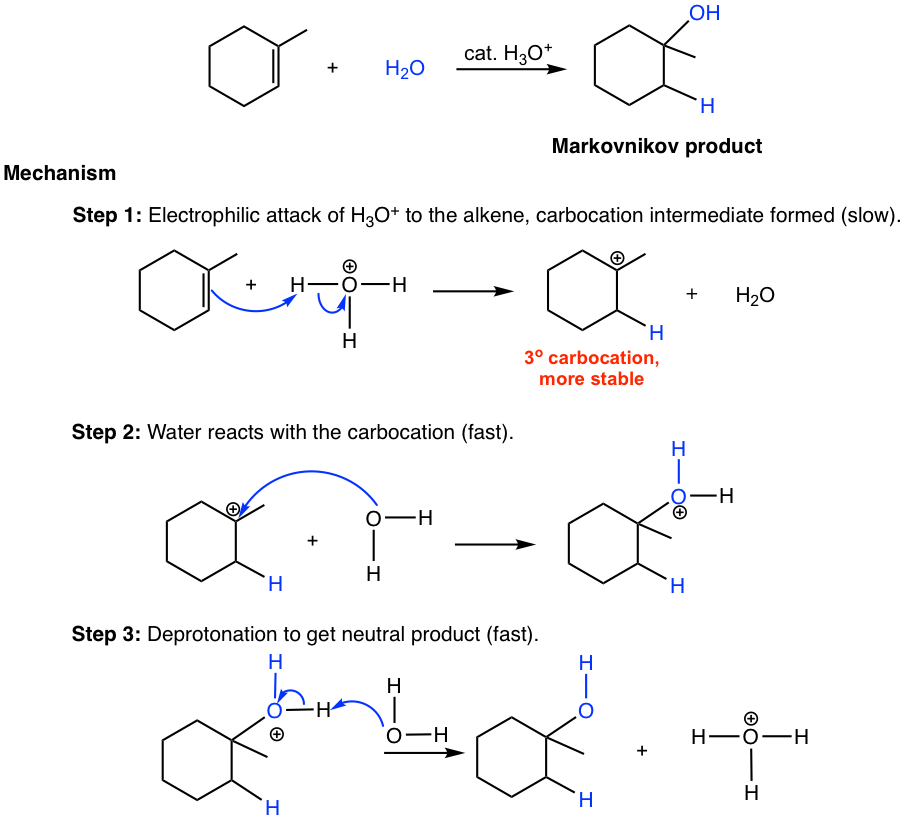
Since a water molecule can be regarded as H—OH, the regioselectivity of alcohol product that follows Markovnikov’s rule means the hydrogen atom connects to the double bond carbon that has more hydrogen atoms, and the OH group adds to the carbon that has fewer hydrogen atoms. This can be explained again by the formation of a more stable carbocation in the first step of the mechanism. The acidic hydronium ion (H3O+) is regenerated in the last deprotonation step, so only a small amount of acid is required to initiate the reaction. The acid therefore is a catalyst.
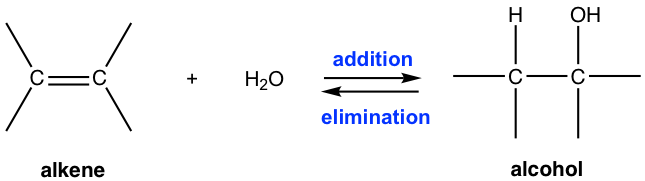
When comparing the hydration reaction of alkene to the dehydration reaction of alcohol in section 10.1.2, you will recognize that they are reverse reactions, as one is addition, and the other is elimination. To produce alcohol from alkene via hydration, water should be in excess to ensure the reaction goes to completion. While preparing alkene from alcohol through dehydration, a high concentration of acid with an elevated temperature favors the elimination process, and the product can be removed by distillation as it is formed to push the equilibrium to the alkene side.
Addition of Alcohol to Alkenes
With the presence of acid, an alcohol can be added to the alkene in the same way that water can be, and ether is formed as a product. For example:

Examples:
Solutions:
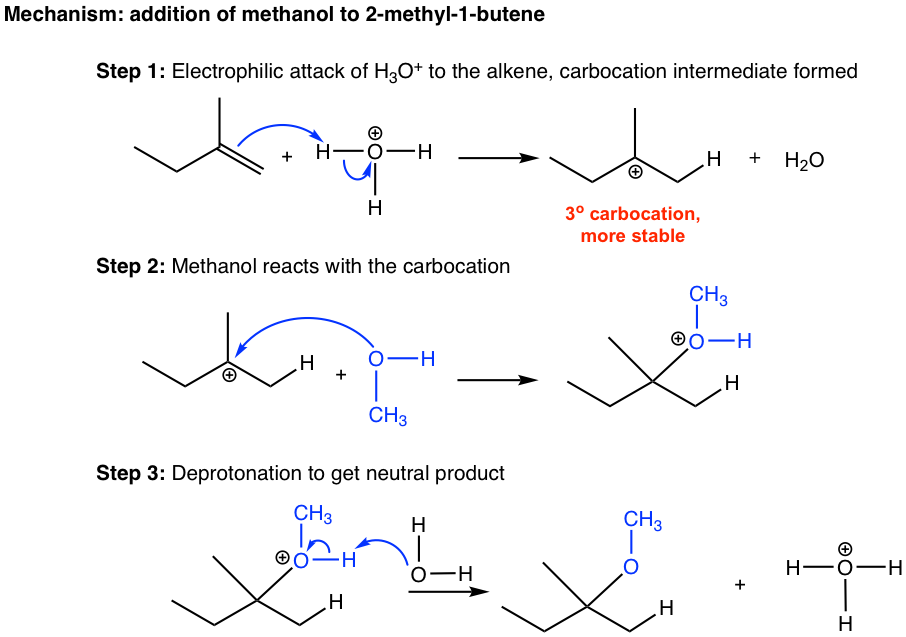
Mechanism: addition of methanol to 2-methyl-1-butene
Step 1: Electrophilic attack of H3O+ to the alkene, carbocation intermediate formed
Step 2: Methanol reacts with the carbocation
Step 3: Deprotonation to get the neutral product
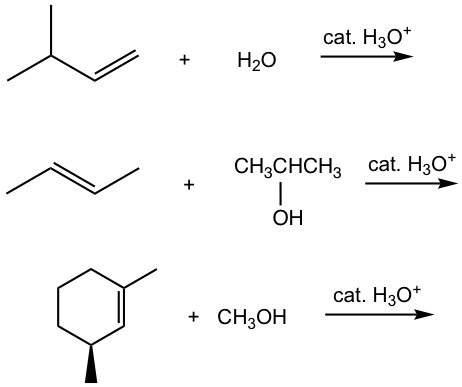
Exercises 10.3
Show major product(s) for the following reactions.