Chapter 7: Nucleophilic Substitution Reactions
7.3 Other Factors that Affect SN2 Reactions
Leaving Group
When alkyl halides undergo nucleophilic substitution reactions, halogen is the leaving group. Not only halogens can be the leaving group, but other appropriate groups can be leaving groups as well. Generally speaking, a nucleophilic substitution reaction requires a good leaving group. The question then is how to decide whether a leaving group is good or not.
When a leaving group departs, it takes the electron pair from the broken bond together with it. So, the good leaving group should be one that can accommodate the electron pair very well, that said the good leaving group should be the group that is stable with the pair of electrons.
The stability of a group with a pair of electrons is related to the basicity of the group since basicity refers to the ability of the species to share its electron pair. As a result, a strong base has high reactivity to share the electron pair, so it is not stable and cannot be a good leaving group. On the other side, a weak base with a low tendency to share the electron pair is a more stable base and therefore is a good leaving group. So, the general trend is:
The weaker the basicity of a group, the better the leaving group is.
Our knowledge of acid-base topics will be very helpful here to compare the strength between different leaving groups.
For alkyl halides, the relative reactivities as a leaving group are:
(best leaving group) I–> Br–> Cl–> F– (weakest leaving group)
This order matches the relative basicity of halide anions. I– is the weakest base and also the best leaving group.
Besides halides, other groups can be leaving groups as well. In the acid-base chapter, we have learned about some examples of strong organic acids, for example, tosylic acid, TsOH, etc. Since the conjugate base of strong acid is a very weak base, the conjugate bases of those acids are good choices for leaving groups as well. Examples include (the leaving group is highlighted in blue):
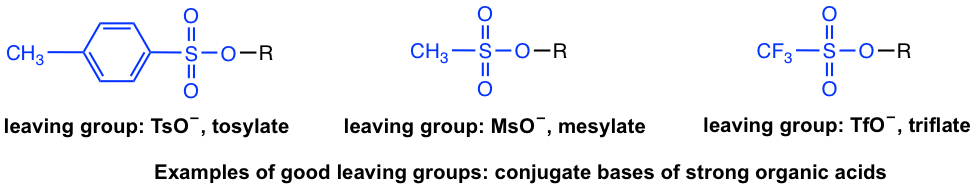
Strong bases such as OH–, RO–, NH2–, and R– are therefore very poor leaving groups and cannot go with nucleophilic substitution reactions. For OH– or RO–, however, upon protonation they can be converted to neutral H2O or ROH molecules, which are leaving groups suitable for substitution. This topic will be covered in section 7.6.
Nucleophile
For an SN2 reaction, the nucleophile is one of the rate-determining factors; therefore, strong nucleophiles help to speed up SN2 reactions.
The relative strength of a nucleophile is called nucleophilicity. The nucleophilicity of a nucleophile is measured in terms of the relative rate of its SN2 reaction with the same substrate. Generally speaking, the nucleophilicity trend depends on several structural features of the nucleophile.
- A nucleophile with a negative charge is always stronger than the corresponding neutral one. For example: OH–> H2O; RO–> ROH.
- Nucleophilicity decreases across a period. For example: NH3 > H2O; RNH2 > ROH
- Nucleophilicity increases across a group. For example:
RSH > ROH; RS–> RO–;
I– > Br– > Cl– > F– (protic solvent)
- A smaller group is a better nucleophile than a bulky group.
For example, t-BuO– 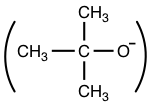 is a very poor nucleophile because of its bulky size.
is a very poor nucleophile because of its bulky size.
To make it more convenient for studying purposes, the commonly applied strong and weak nucleophiles are listed here:
Strong (good) nucleophile: OH–, RO– (small alkoxide), RS–(thiolate), N3–(azide), CN–(cyanide), Cl–, Br–, I–(halide), RCO2–(carboxylate), RNH2 (amine)
Weak (poor) nucleophile: ROH, H2O, t-BuO–
With the structure of nucleophiles being so diverse, SN2 reactions can be used to synthesize compounds with a variety of functional groups, as shown here.
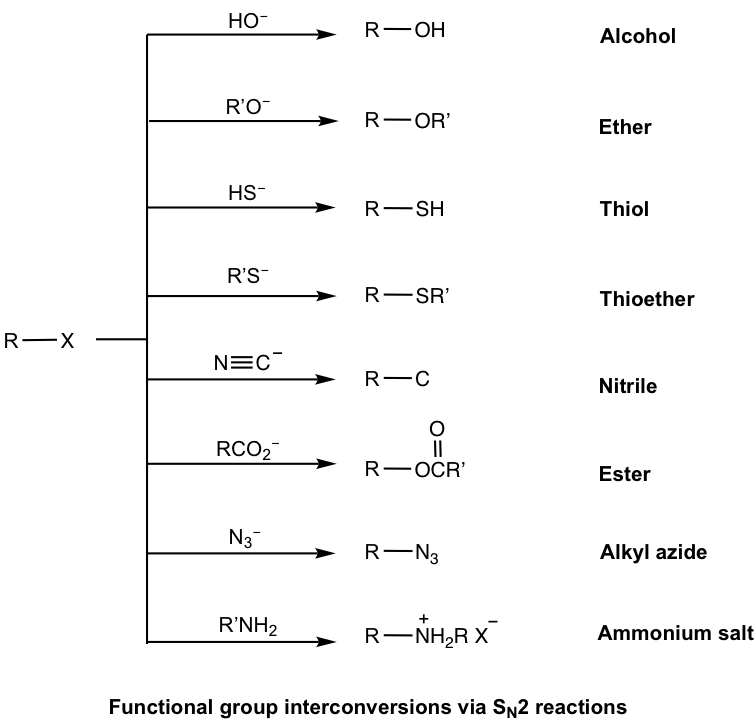
Examples
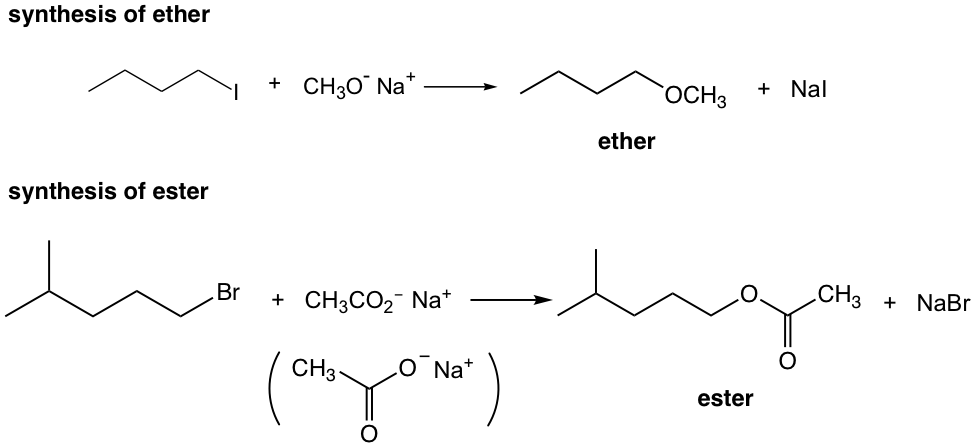
Exercises 7.2
Show the reaction mechanism of the above reactions.

