Chapter 5: Stereochemistry
5.3 Chirality and the R/S Naming System
Other than geometric isomers, there is another type of stereoisomer that is related to a special property called chirality. We will start with the basic concepts of chirality, and then expand the topic further from there.
5.3.1 Chiral and Chirality
To talk about chirality, let’s first take a closer look at our left hand and our right hand. The left hand can be regarded as a mirror image of the right hand, and vice versa. Now, let’s try to superimpose (overlay) the left hand on the right hand. Can you do that?
No! No matter how hard you try, the left hand can not be superimposed on the right hand. This is because of the special property of the hand that is called chirality. Both the left and right hand are chiral (ky-ral) and show chirality. Chiral is derived from the Greek word cheir, which means “hand”, and chirality means “handedness”.
The definition of chirality is the property of any object (molecule) being non-superimposable on its mirror image. The left and right hand are mirror images of each other, and they are not superimposable, so both the left hand and right hand are chiral. You can also find many other objects in daily life that show chirality as well.

If an object is superimposable on its mirror image (in such a case, the object and its mirror image are exactly identical), then this object is not chiral, and it is referred to as achiral.
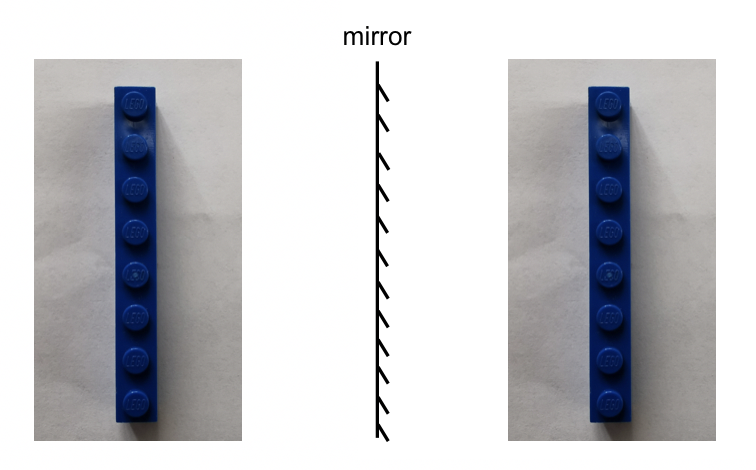
In organic chemistry, we are interested in organic molecules that are chiral. Let’s see the following molecular models that represent a molecule and its mirror image.
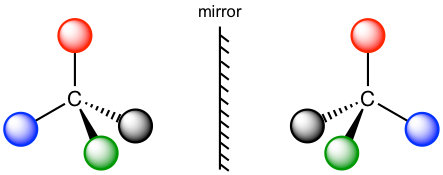
In the models here, the four balls with different colors represent four different substituents, and the two structures are mirror images of each other. The effort of trying to superimpose one structure on the other does not work. Therefore, according to the definition of chiral/chirality, both molecules are non-superimposable on the mirror image, so they are both chiral and show chirality.
The chirality of the molecule results from the structure of the central carbon. When the central carbon is sp3 carbon and bonded with four different groups (represented by four different colors in the model), the molecule is chiral. The central carbon is called the chirality center (or asymmetric center). A molecule with one chirality center must be chiral. The chirality center can also be called the asymmetric center. We will use the term chirality center in this book.
In summary, a chirality (asymmetric) center should meet two requirements:
- sp3 carbon;
- bonded with four different groups.
Examples
For following compounds, label each of the chirality center with a star.
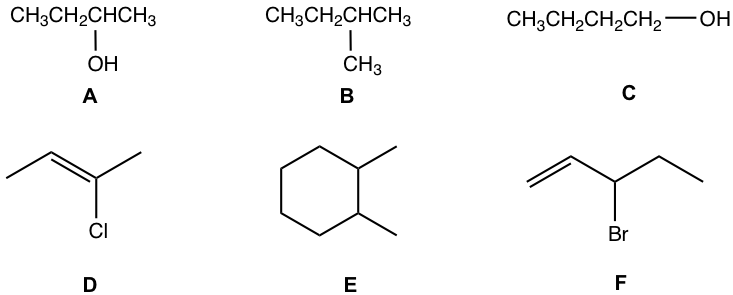
Approach:
- The carbons in CH3 or CH2 are NEVER chirality centers. The chirality center must be the carbon bonded with a branch (or branches).
- sp2 double bond carbon is NEVER a chirality center.
- Carbon in a ring can also be chirality center as long as it meet the two requirements.
- Not all the above compounds have a chirality center.
Solution:

Exercises 5.2
- Draw the structure of the following compounds, determine which one has a chirality center, and label it with a star.
a) 1-bromobutane,
b) 1-pentanol,
c) 2-pentanol,
d) 3-pentanol,
e) 2-bromopropanoic acid
f) 2-methyl cyclohexanone
2. Label all the chirality centers in the following molecules.
Answers to Chapter 5 Practice Questions
5.3.2 Stereoisomer with One Chirality Center — Enantiomers
For 2-butanol, we can recognize that C2 is the chirality center.

The perspective formula shows the 3D structure of 2-butanol in two different ways, and they are non-superimposable mirror images of each other.
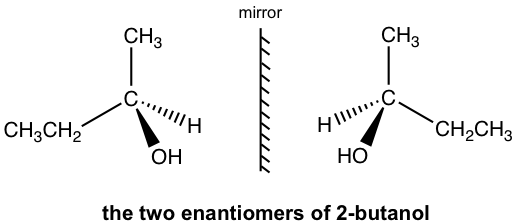
The two mirror images are different molecules. They have the same bonding but differ in the way the atoms are arranged in space. So, the two molecules are stereoisomers. This specific type of stereoisomer is defined as an enantiomer. Molecules that are a pair of non-superimposable mirror images of each other are called enantiomers.
Important Properties of Enantiomers:
- Enantiomers are a pair of non-superimposable mirror images.
- Enantiomers are a pair of molecules that are both chiral and show chirality (Enantiomers must be chiral).
- For any chiral molecule, it must have its enantiomer, that is, the mirror image of the molecule.
- Achiral molecules do not have enantiomers. The mirror image of an achiral molecule is an identical molecule to itself.
Approach:
To draw the 3D structure of any enantiomer, we need to use a perspective formula with solid and dashed wedges to show the tetrahedral arrangements of groups around the sp3 carbon (refer to section 2.11). Out of the four bonds on tetrahedral carbon, two bonds lie within the paper plane are shown as ordinary lines, the solid wedge represents a bond that points out of the paper plane, and the dashed wedge represents a bond that points behind the paper plane. For the first enantiomer, you can draw the four groups with any arrangement, then draw the other enantiomer by drawing the mirror image of the first one. Please note, that although it seems there are different ways to show the enantiomers, there are only a total of two enantiomers, we will learn in the next section how to identify and designate each of them.
Several possible ways to show the structures are included in the answer here. However, your answer can be different from any of them, as long as a pair of mirror images is shown.
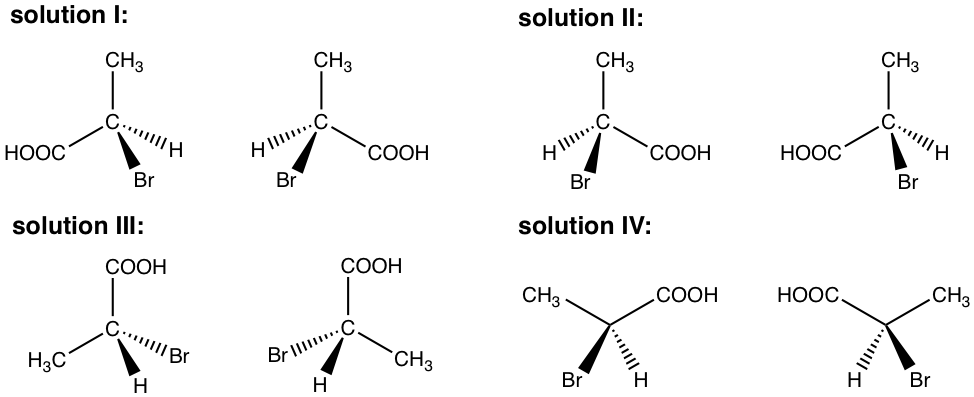
Exercises 5.3
Draw the pair of enantiomers of 2-chloro-1-propanol.
Answers to Chapter 5 Practice Questions
5.3.3 R/S Naming System of the Chirality Center
The two enantiomers are different compounds, though they are very similar; therefore, we need a nomenclature system to distinguish between them, to give each one a different designation so that we know which one we are talking about. That is the R/S naming system defined in IUPAC. The R/S designation can be determined by following the Cahn-Ingold-Prelog rule, the rule devised by R. S Cahn, C. Ingold, and V. Prelog.
Cahn-Ingold-Prelog Rule:
- Assign priorities of the groups (or atoms) bonded to the chirality center by following the same priority rules as for the E/Z system (section 5.2). The highest priority group is labeled as #1, and the lowest priority group is labeled as #4 in this book.
-
Orient the molecule in a way that the lowest priority group (#4) is pointing away from you.
-
Look at the direction in which the priority decreases for the other three groups, that is 1→2→3.
For the counterclockwise direction, the designation is S–, sinister, which means “left” in Latin.
Let’s take the following molecule as an example to practice the rule:
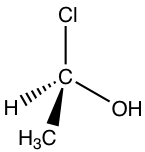
Step 1: The priorities are assigned.
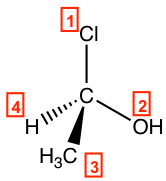
Step 2: Re-orient the molecule, so H (#4, lowest priority) is in the position away from us. Then, the other three groups will be arranged in this way:
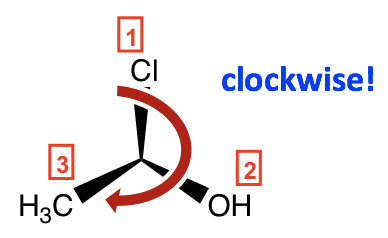
Step 3: Go along the direction from #1→#2→#3; it is in the clockwise direction, so this enantiomer is assigned an R configuration, and the complete name of the molecule is (R)-1-chloroethanol.
Now, let’s assign the configuration of the other enantiomer:
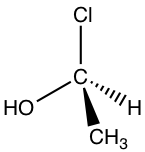
Following the same steps, put H away from us, and the arrangement of the other three groups is:
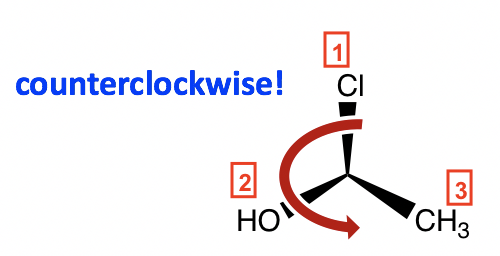
The counterclockwise direction gives the S configuration, and the complete name of the molecule is (S)-1-chloroethanol.
Examples: Assign R/S configuration of the chirality center.
1.
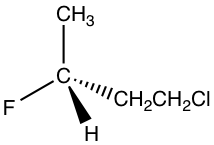
Solution:

2.
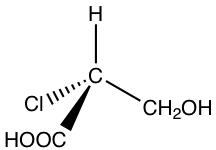
Solution:

More practical hints about R/S assignment with the Cahn-Ingold-Prelog rule:
- Assigning priority is the first possible challenge for applying the C.I.P. rule. Review and practice the guidelines in section 5.2.
- The second challenge is to re-orient the molecule (to arrange the #4 group away from you). The molecule model will be very helpful for this purpose. Assemble a molecular model with four different colors connected to the carbon. Compare your model to the given structure and match the assigned priority to each color; for example, red is #1, blue is #2, etc. Then, rotate the model to arrange the lowest (#4) group away from you and see how the other groups are located to get the answer.
For the perspective formula of enantiomers, it is important to know the following properties:
- One (odd number of) switch (interchanging) for a pair of groups inverts the configuration of the chirality center.
- Two (even number of) switches get the original configuration back.
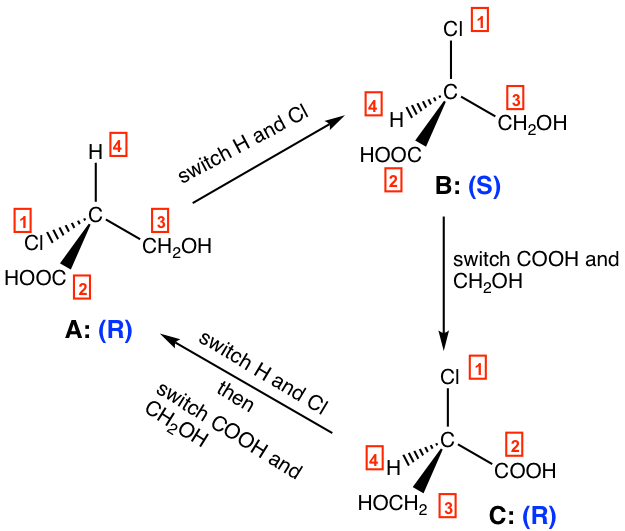
For the structures above:
- One switch of A leads to B, A is R and B is S, so A and B are enantiomers.
- One switch of B leads to C, B is S and C is R, so B and C are enantiomers.
- Two switches of C lead to A, and both C and A are R, so C and A are identical.
Exercises 5.4
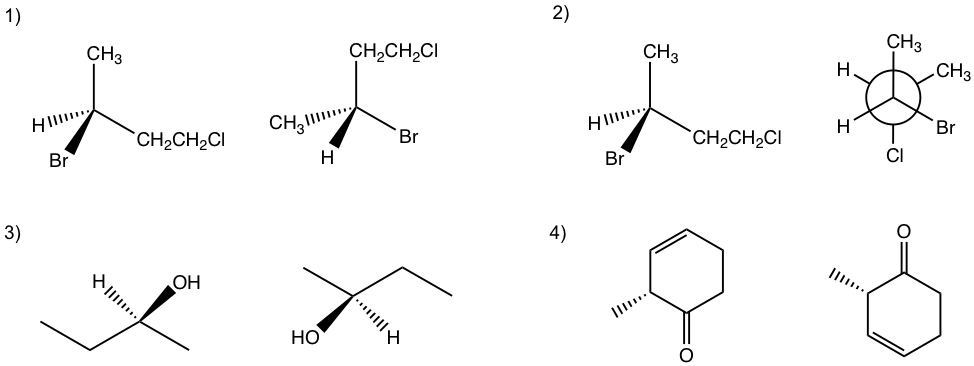
Determine the R/S configuration of the chirality center in following compounds.
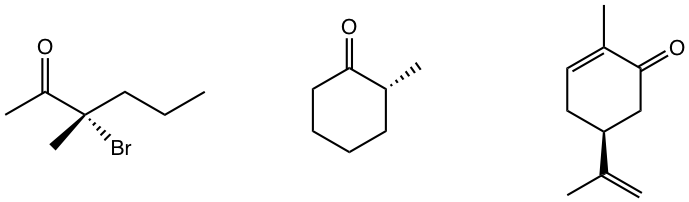
Answers to Chapter 5 Practice Questions
Exercises 5.5
Determine the relationship for each pair of molecules: enantiomers, identical, constitutional isomers, non-isomer: