Chapter 6: Structural Identification of Organic Compounds: IR and NMR Spectroscopy
6.8 ¹³C NMR Spectroscopy
For a carbon element, the most abundant isotope 12C (with ~99% natural abundance) does not have a nuclear magnetic moment and thus is NMR-inactive. The C NMR is therefore based on the 13C isotope, which accounts for about 1% of carbon atoms in nature and has a magnetic dipole moment just like a proton. The theories we have learned about 1H NMR spectroscopy also apply to 13C NMR, but with several important differences in the spectrum.
The magnetic moment of a 13C nucleus is much weaker than that of a proton, meaning that 13C NMR signals are inherently much weaker than proton signals. This, combined with the low natural abundance of 13C, means that it is much more difficult to observe carbon signals. Usually, samples with a high concentration and large number of scans (thousands or more) are required to bring the signal-to-noise ratio down to acceptable levels for 13C NMR spectra.
Chemical Equivalent
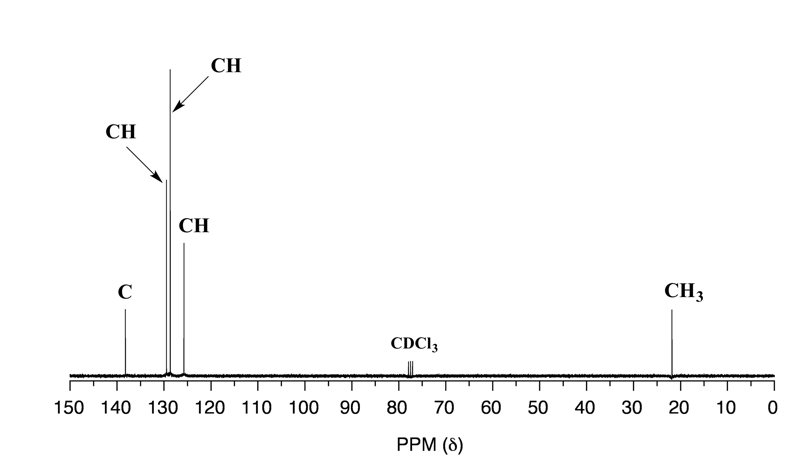
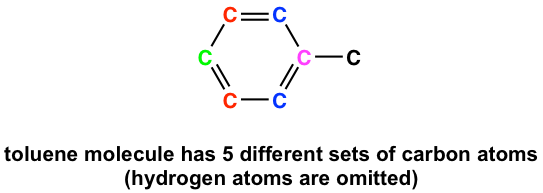
For carbons that are chemical equivalent, they only show one signal in 13C NMR, similar to protons for 1HNMR. Considering this, it is very important to be able to identify equivalent carbons in the structure to interpret the 13C NMR spectrum correctly. Taking toluene as an example, there are five sets of different carbon atoms (shown in different colors), so there are five signals in the 13C NMR spectrum of toluene.
Chemical Shift
13C nuclei have a different value of g (the magnetogyric ratio) compared to 1H nuclei, so the resonance frequencies of 13C nuclei are different from those of protons in the same applied field (referring to formula 6.4, in section 6.5). In an instrument with a 7.05 Tesla magnet, protons resonate at about 300 MHz, while carbons resonate at about 75 MHz. This allows us to look at 13C signals using a completely separate ‘window’ of radio frequencies. Just like in 1H NMR, tetramethylsilane (TMS) is also used as the standard compound in 13C NMR experiments to define the 0 ppm; however, it is the signal from the four equivalent carbon atoms in TMS that serves as the standard. Chemical shifts for 13C nuclei in organic molecules are spread out over a much wider range of about 220 ppm (see Table 6.3).
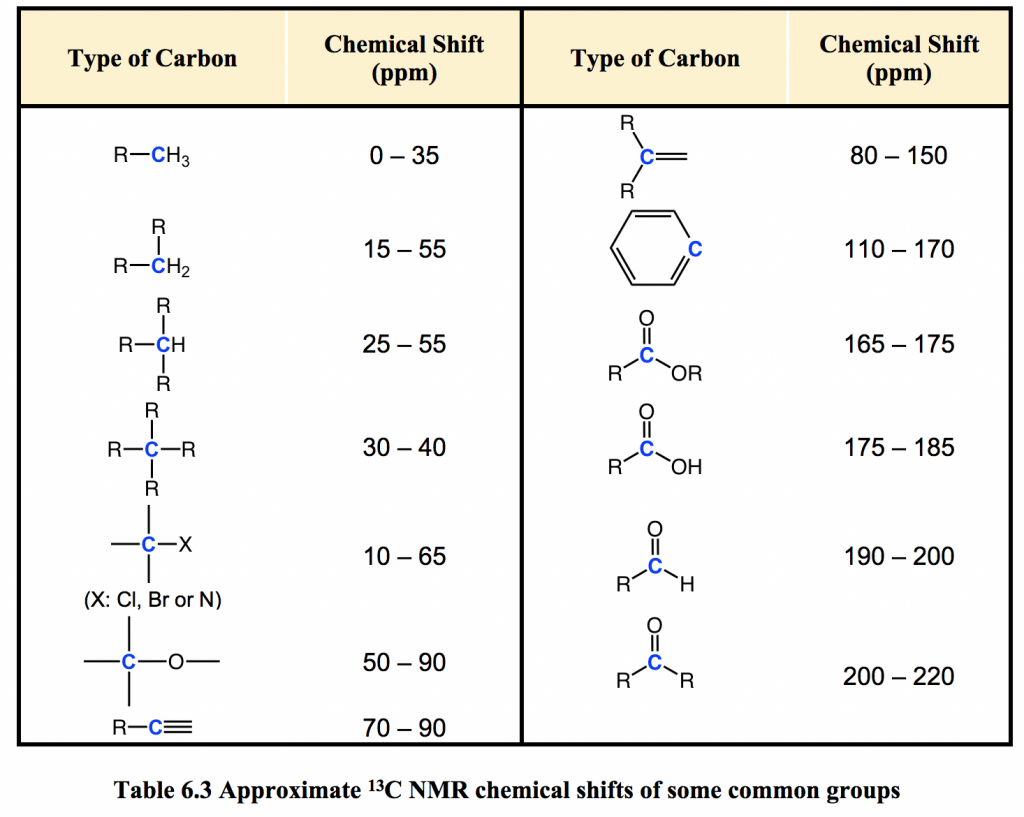
The chemical shift of a 13C nucleus is influenced by essentially the same factors that influence the chemical shift of a proton: the deshielding effect of electronegative atoms and anisotropy effects tend to shift signals downfield (higher resonance frequency, with higher chemical shifts). In addition, sp2 hybridization results in a large downfield shift. The 13C NMR signals for carbonyl carbons are generally the furthest downfield (170–220 ppm) due to both sp2 hybridization and the double bond to oxygen.
Integration and Coupling in 13C NMR
Unlike 1H NMR, the area under a 13C NMR signal cannot easily be used to determine the number of carbons to which it corresponds. The signals for some types of carbons are inherently weaker than for other types; for example, peaks corresponding to carbonyl carbons are much smaller than those for methyl or methylene (CH2) peaks. For this reason, signal integration is generally not useful in 13C NMR spectroscopy.
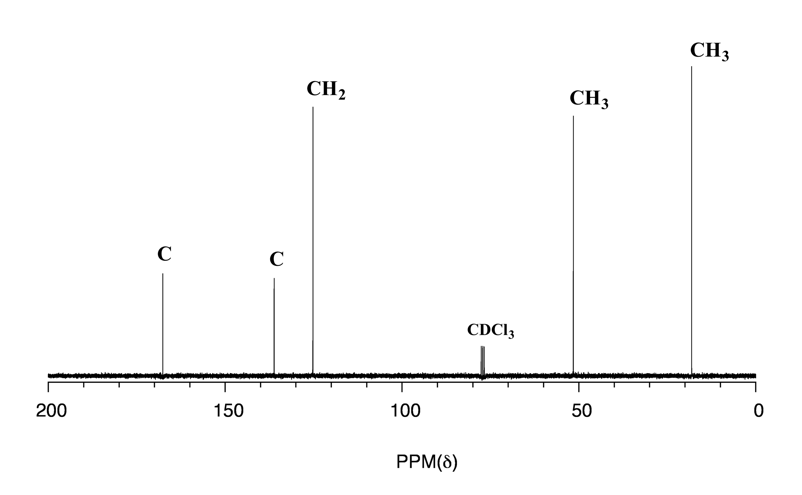
Because of the low natural abundance of 13C nuclei, the spin-spin coupling between two nonequivalent 13C atoms is negligible. 13C nuclei are coupled to nearby protons, which results in complicated spectra. For clarity, chemists generally use a technique called broadband decoupling, which essentially ‘turns off’ C-H coupling, resulting in a spectrum in which all carbon signals are singlets. Below is the proton-decoupled 13C NMR spectrum of ethyl acetate in CDCl3 (Fig. 6.8a), showing the expected four signals, one for each of the carbons.
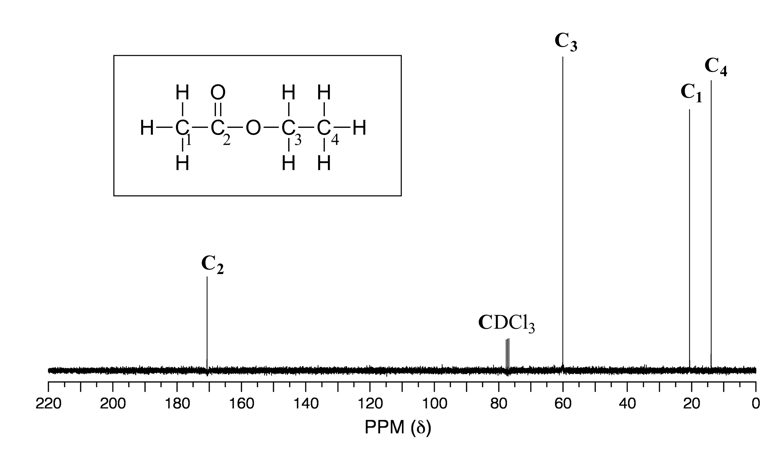
For our purposes, 13C NMR spectra are usually used as supporting information to confirm the structure of a compound.
Exercises 6.2