Chapter 9: Free Radical Substitution Reaction of Alkanes
9.5 Stereochemistry for the Halogenation of Alkanes
In the structure of a carbon radical, carbon has three bonds and one single electron. Based on VSEPR, there are a total of four electron groups, and the radical should be in a tetrahedral shape. However, experimental evidence indicates that the geometric shape of most alkyl radicals is a trigonal planar shape, with the carbon in sp2 hybridization, and there is one single unpaired electron in the unhybridized 2p orbital.
We will take the bromination reaction of (±)-3-methylhexane to explain the stereochemistry. The experiment results indicate that the racemic mixture of R and S 3-bromo-3-methylhexane was obtained with the bromination.
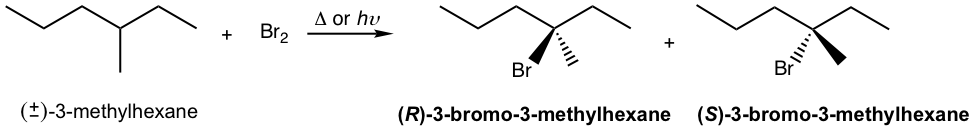
This can be explained by the stereochemistry of the propagation steps in the mechanism. The carbon radical generated in step 1 is in a trigonal planar shape as mentioned earlier. When the radical reacts with bromine in step 2, the reaction can occur at either side of the plane. Because both sides are identical, the probability of the reaction by either side is the same; therefore, an equal amount of the R– and S– enantiomers are obtained as a racemic mixture.
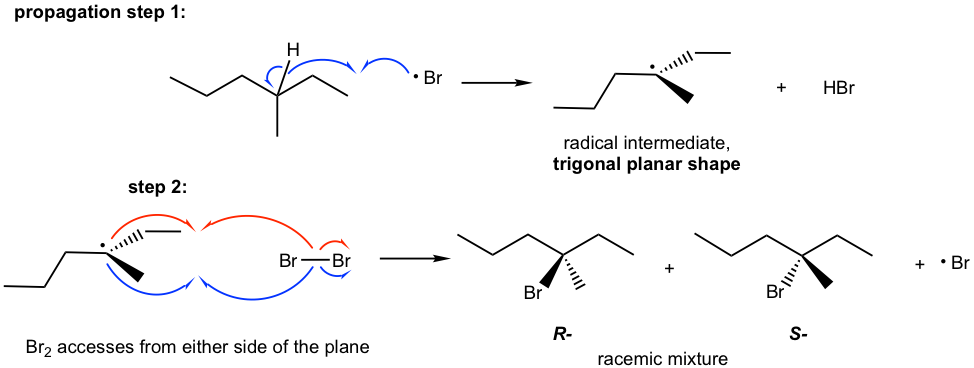
The stereochemistry of the radical substitution is similar to that of the SN1 reaction because both carbon radical and carbocation are in a trigonal planar shape.
Examples
Show the bromination product(s) with stereoisomers when applied.
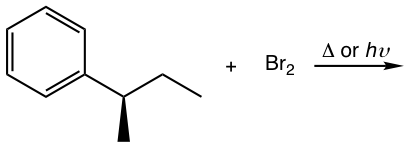
Solutions:
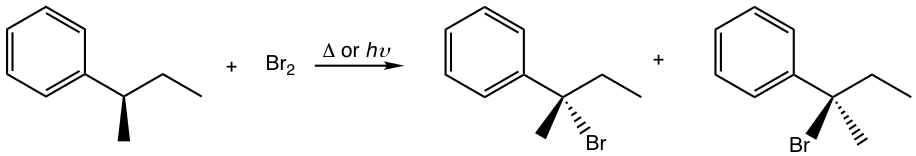
The racemic mixture is obtained.
Exercises 9.3
Show all the mono-chlorination products of butane with any stereoisomers when applied.
![]()

