Chapter 10: Alkenes and Alkynes
10.2 Reactions of Alkenes: Addition of Hydrogen Halide to Alkenes
Alkenes undergo a wide variety of reactions. At first glance, these reactions appear to be quite different, but detailed studies indicate that the different mechanisms all share some common features. The double bond is the reactivity center of alkene; this is mainly because of the relatively loosely held π electrons of the double bond. The π bond is formed by side-by-side overlapping, a relatively weak overlapping mode, so the π bond is weak and exhibits high reactivity. The π electrons also make the double bond carbons electron-rich and have the tendency to be attracted to an electrophile. The high reactivity makes alkenes an important type of organic compound, and they can be used to synthesize a wide variety of other compounds, such as halides, alcohols, ethers, and alkanes.
The most common type of reaction for alkene is the addition reaction to a C=C double bond. In addition reaction, a small molecule is added to multiple bonds, and one π bond is converted to two σ bonds (unsaturation degree decreases) as a result of the addition. An addition reaction is the opposite process of elimination.
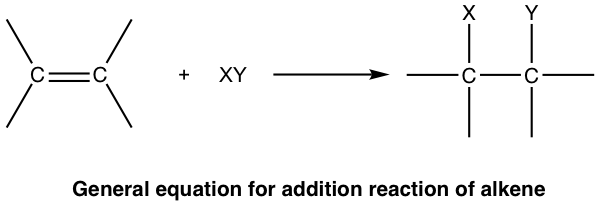
The addition reactions can generally be categorized depending on what small molecule is added, our following discussions will also be based on that.
10.2.1 Addition of Hydrogen Halide to Alkenes
The addition reaction of a hydrogen halide to an alkene produces an alkyl halide as a product. For examples:


In the above reactions, the alkenes are in symmetric structures, which means it does not matter which carbon bonded with hydrogen and which carbon bonded with the halogen, as the same product will be obtained either way.
For the alkene that does not have a symmetric structure, the double bond carbons have different substituents so the question of which carbon gets the hydrogen is critical. For the example of the following reaction, two possible products could be produced: 2-bromo-2-methylpropane and 1-bromo-2-methylpropane. Which one is actually formed? Or are both formed?
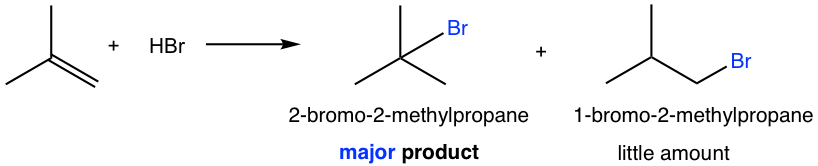
It turns out that 2-bromo-2-methylpropane is the main product of the reaction. To explain and understand the outcome of the reaction, we need to look at the mechanism of the reaction.
The mechanism of the addition reaction involves two steps (shown below). In the first step, the π electrons of the alkene act as nucleophiles and are attracted to the partially positively charged hydrogen (electrophile) of HBr. As the π electrons of the alkenes move toward the hydrogen, the H-Br bond breaks, Br moves away with the bonding electrons, and a new σ bond is formed between one double bond carbon and hydrogen. A carbocation and a bromide, Br–, are formed in this step.
In the second step, the bromide, Br–, reacts with the positively charged carbocation to give the final product. This step is similar to the second step of the SN1 reaction, in which a nucleophile reacts with an electrophile (carbocation).
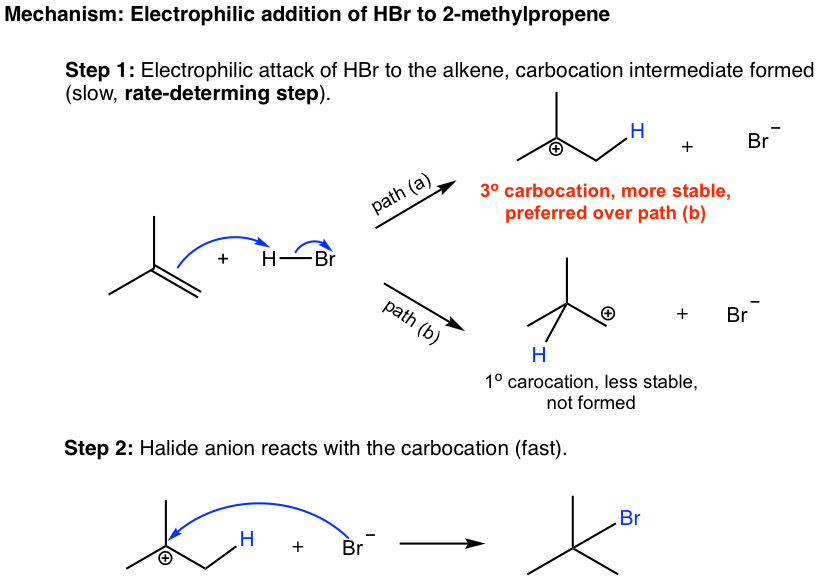
When the new bond is formed between the double bond carbon and hydrogen in the first step, the hydrogen could possibly be bonded with either carbon, as shown in paths (a) and (b), and carbocations with a different structure will be produced. It is obvious that the tertiary carbocation formed in the path (a) is much more stable than the primary carbocation in the path (b) and will be produced preferably. The tertiary carbocation is then attacked by the Br– in the second step, which produces the product 2-bromo-2-methylpropane. It is the stability difference between two carbocations in the first step that accounts for the selective formation of 2-bromo-2-methylpropane in the overall reaction.
Because the first step of the above reaction is the addition of an electrophile (H+) to the alkene, the reaction is called an electrophilic addition reaction. An electrophilic addition reaction is a characteristic type of reaction of alkenes, and several other addition reactions, as we will see later, also belong to this category.
The two possible products of this reaction are constitutional isomers to each other. For the reaction in which two or more constitutional isomers could be obtained as products, but one predominates, the reaction is said to be a regioselective reaction. Regio comes from the Latin word regionem, which means direction. The regioselectivity trend of the electrophilic addition of HX to alkenes was summarized as Markovnikov’s rule by Russian chemist Vladimir Markovnikov. One way to state Markovnikov’s rule is that in the addition of HX to an alkene, the hydrogen atom adds to the double bond carbon that has the greater number of hydrogen atoms.
The underlying reasoning for Markovnikov’s rule is the stability of the carbocation intermediate involved in the reaction mechanism. It may seem easy to just memorize the rule or memorize the fact that 2-bromo-2-methylpropane is the product of the above reaction without understanding why. However, you would quickly notice that your memorization would be overwhelmed and mixed up with the many reactions coming up. The proper way to study organic reactions is to learn and understand the mechanism and unify the principles of the reactions based on mechanisms. The mastery of the contents will be much easier and a lot more fun in this way.
Exercises 10.1
Answers to Chapter 10 Practice Questions
Exercises 10.2
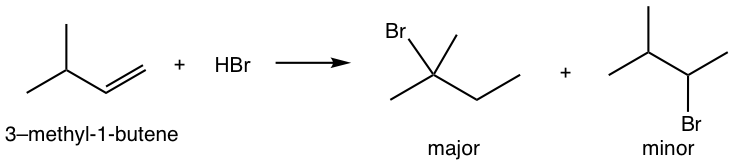
For the addition of HBr to 3-methyl-1-butene, two products were observed. Show the reaction mechanism to explain the formation of both products.
10.2.2 Radical Addition of HBr to Alkenes
In the last section, we learned that the electrophilic addition of HX to alkene gives addition products that follow Markovnikov’s rule. Here, we will learn that hydrobromide, HBr, can also be added to alkene in a way that gives an anti-Markovnikov product.
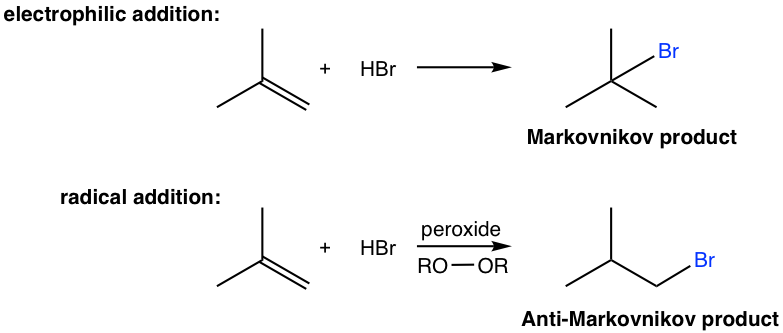
The anti-Markovnikov product is obtained through a different mechanism, which is the radical mechanism. To initiate the radical mechanism, peroxide must be involved to generate the radical in the initiation step of the mechanism. The O-O bond of peroxide is weak (with a bond energy of about 150 kJ/mol), and it undergoes homolytic cleavage readily with heat to produce alkoxyl radicals. The peroxide therefore acts as a radical initiator by generating radicals, and the addition is called radical addition. The detailed radical addition mechanism of the above addition of HBr to 2-methylpropene is given here.
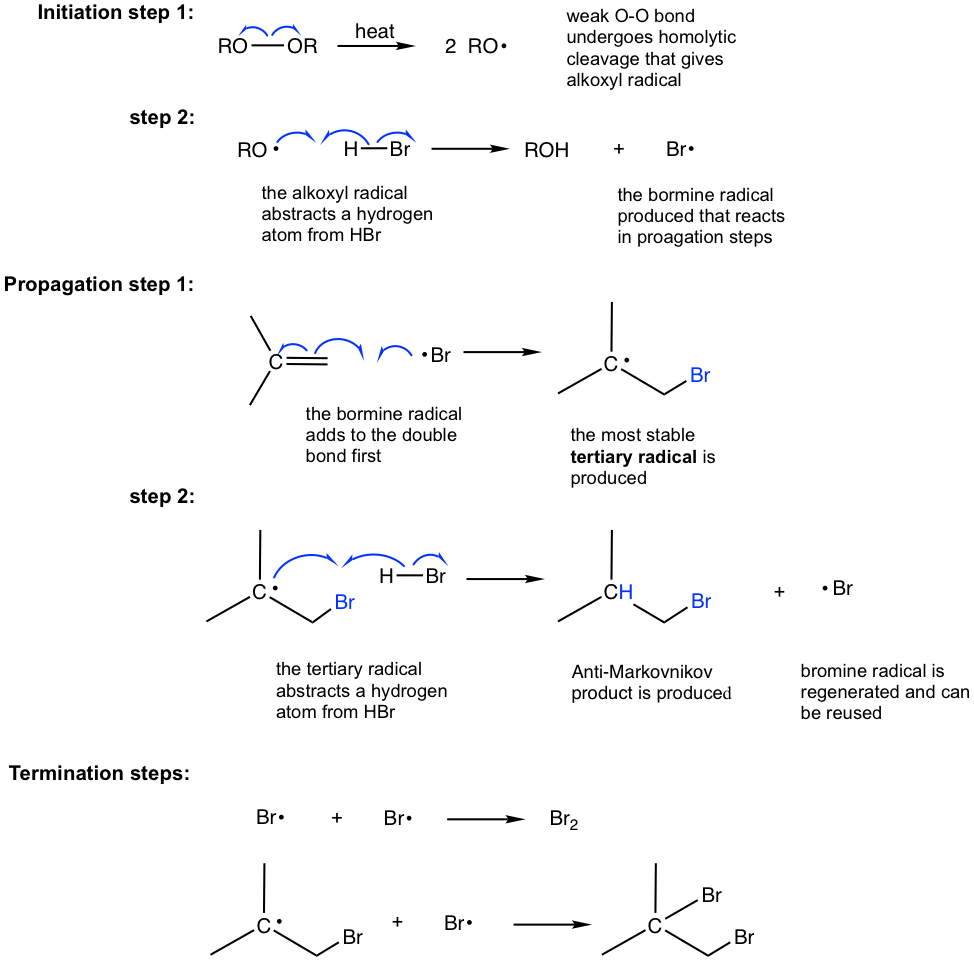
The initiation involves two steps for the radical addition mechanism. The alkoxyl radical generated in step 1 reacts with H-Br to generate the bromine radical, Br·, which reacts with an alkene to initiate the chain reaction in the propagation steps. It is shown clearly in the propagation steps that the order of the addition is reversed in radical addition compared to that of electrophilic addition. Specifically, the bromine radical (Br) is added to the double bond first followed by the abstraction of hydrogen atoms (H); therefore, the anti-Markovnikov product is produced as a result.
One more note is that only HBr proceeds with radical addition in the presence of peroxide, not HCl or HI.

