Chapter 4: Conformations of Alkanes and Cycloalkanes
4.4 Substituted Cyclohexanes
Monosubstituted cyclohexane
For the cyclohexane ring itself, the two conformers from the ring flipping are equivalent in terms of energy since there are always six hydrogens in the axial position and six hydrogens in the equatorial position. For substituted cyclohexane, however, the two chair conformations are not equivalent anymore. Let’s see the example of methylcyclohexane.
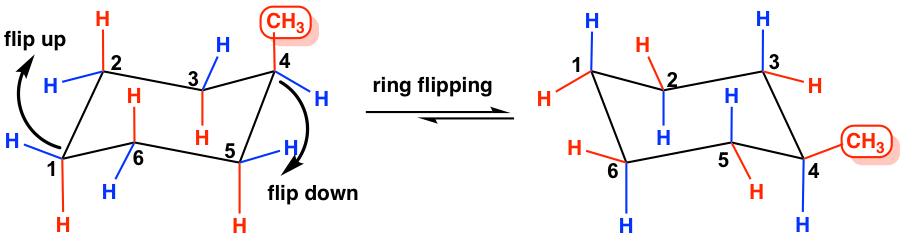
Methylcyclohexane has two chair conformations that are interconvertible through the ring flipping. In conformation I, the methyl group occupies an axial position, and in conformation II the methyl group occupies an equatorial position. Studies indicate that the conformer II with the equatorial-methyl is more stable, with an energy of about 7.6 kJ/mol lower than the other conformer.
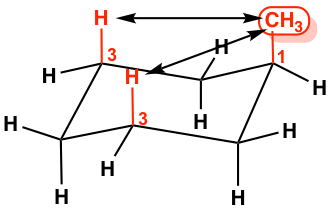
This difference is due to the “1,3-diaxal interaction”. In axial–methyl conformation, the methyl CH3 group (regarded as #1 position) is very close to the axial hydrogens that are one carbon away (regarded as #3 position), and it causes repulsion between the two, which is called the 1,3-diaxal interaction. This type of repulsion is essentially the same as the gauche steric strain because the CH3 group and the CH are in a gauche position. For the equatorial-methyl conformer, no such strains are applied because the CH3 group and the CH are in an anti-position. This interaction can be illustrated more clearly by a Newman projection.
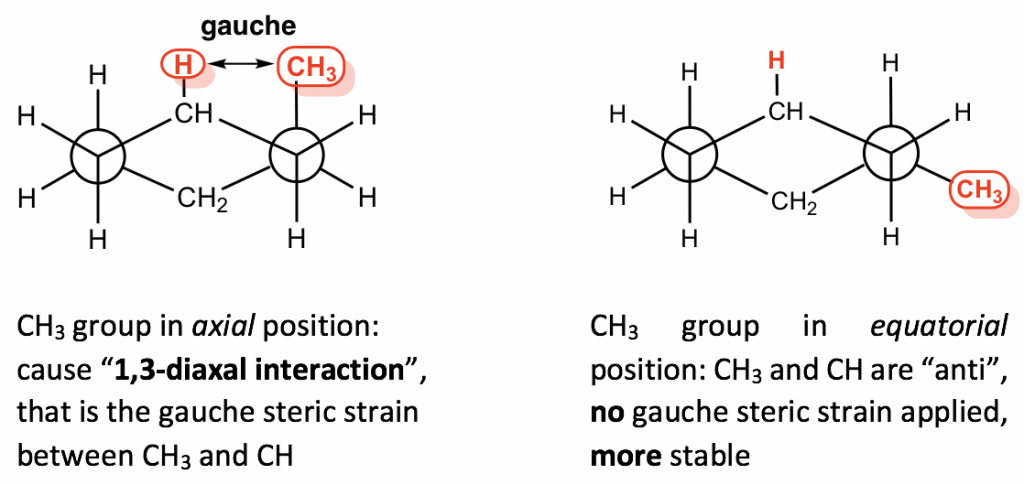
Since the 1,3-diaxal interaction is essentially the steric strain, the larger the size of the substituent, the greater the interaction is. For t-butylcyclohexane, the conformation with the t-butyl group in the equatorial position is about 21 kJ/mol more stable than the axial conformation.
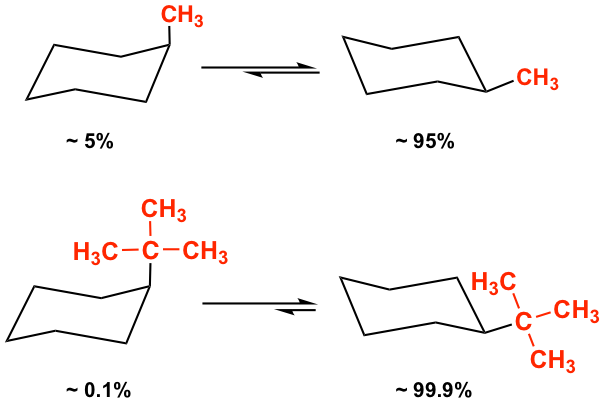
Because of the stability difference between the two chair conformers, the equatorial conformation is always the predominant one in the equilibrium mixture. The larger the size of the substituent, the larger the energy difference and the equilibrium constant K, so the equilibrium lies more toward the “equatorial” side. For methylcyclohexane, there is about 95% equatorial conformer in the mixture, and the percentage is about 99.9% for t-butylcyclohexane.
Disubstituted cyclohexane
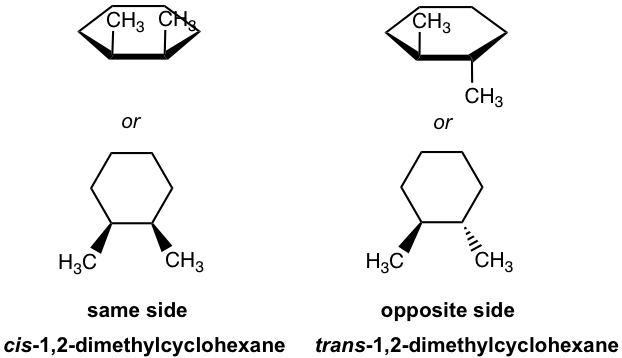
When there are two substituents on different carbons of a cycloalkane, there are two possible relative positions between the two groups – they can be either on the same side or the opposite side of the ring – that are called geometric isomers, a type of stereoisomer (further discussed in Chapter 5). The isomer with two groups on the same side of the ring is the “cis” isomer, and the one with two groups on the opposite side is called the “trans” isomer. Because the C-C bond can not rotate freely due to the restriction of the ring, the two geometric isomers can not be interconverted.
So now when considering the conformational isomer, the stereoisomers should be taken into account as well. The general guideline for determining the relative stability of conformers for a certain isomer is:
- The steric effects of all substituents are cumulative, and the more substituents in equatorial positions, when possible, the more stable the conformation isomer will be.
- For different substituents, the conformer with the larger substituent in the equatorial position is more stable.
Let us start with cis-1,2-dimethylcyclohexane and compare between the two possible chair conformations:
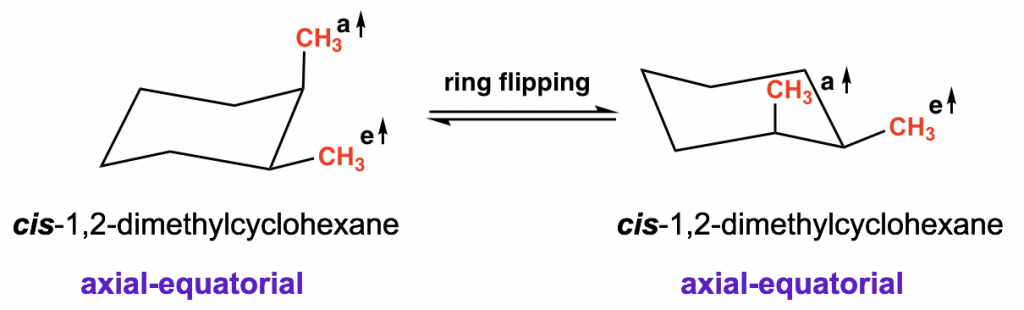
For both conformations, there is one methyl group in the equatorial and the other methyl group in axial, so the two conformers are equivalent and have the same energy and stability level.
How can you tell whether an isomer in the chair conformation is cis or trans? A general way to recognize this is to check whether a group attached by the bond is above the ring (↑, point up) or below the ring (↓, point down). If both groups point to the same side, the compound is a cis isomer; otherwise, it is a trans isomer.
How about the trans-1,2-dimethylcyclohexane? There are also two possible chair conformations:
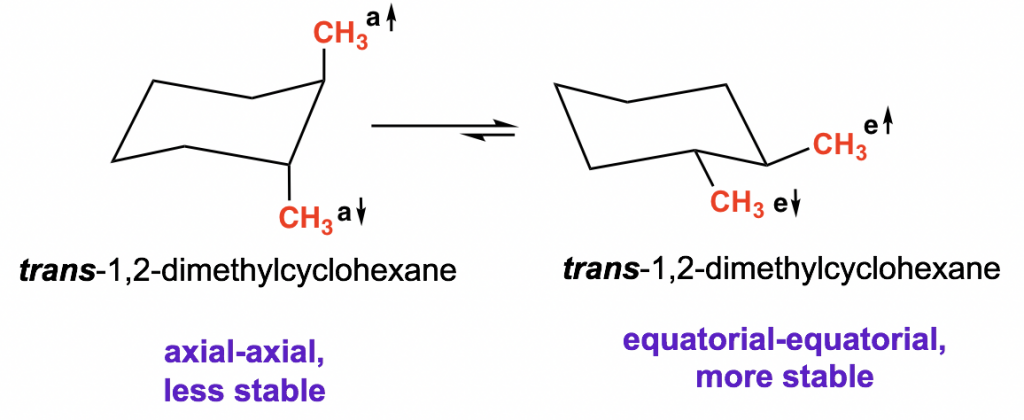
In one conformation both methyl groups are axial, and in the other conformation, both methyl groups are equatorial. These two conformers are not equivalent, and the di-equatorial one is the more stable conformation as we would expect.
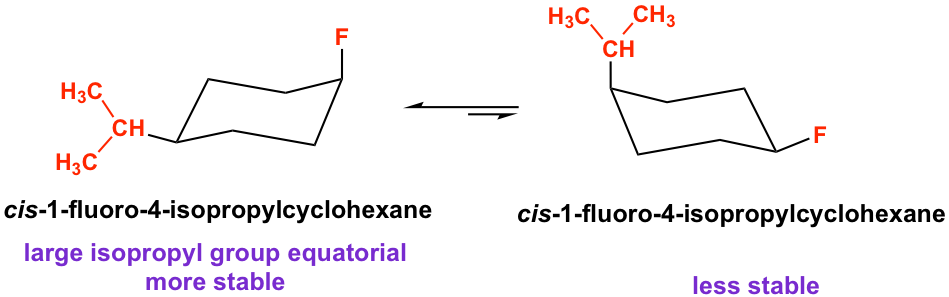
cis-1-fluoro-4-isopropylcyclohexane is the structure with two different substituents. Both chair conformations have one axial substituent and one equatorial substituent. According to the guideline, the conformer with the larger substituent in equatorial is more stable because if the large group is axial, a stronger steric strain will be generated and it is less stable.
Exercises 4.3
Determine which is the more stable isomer, cis-1-ethyl-2-methylcyclohexane or trans-1-ethyl-2-methylcyclohexane?
Tips: draw all the chair conformers of each isomer, and decide which is the most stable one.

