Chapter 3: Acids and Bases: Introduction to Organic Reaction Mechanism Introduction
3.5 Lewis Acids and Lewis Bases
The Brønsted-Lowry definition works well for the reactions we have learned so far, but it also limits the scope of acid-base reactions in a way in which the proton H+ must be involved. Lewis acids and Lewis bases are defined in a more inclusive way that was first introduced by G.N. Lewis in 1923.
Lewis Acid: a species that can accept an electron pair
Lewis Base: a species that can donate an electron pair
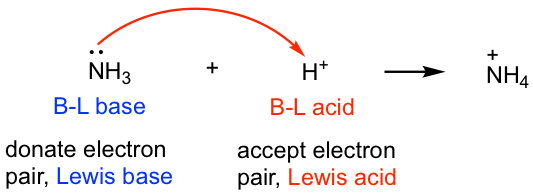
All Brønsted-Lowry acids and bases fit into the Lewis definition because the proton transfer process is essentially the reaction where the base uses its electron pair to accept a proton, as indicated by the mechanism arrow that we learned about earlier. Therefore in the following reaction, the BL acid, H+, is also the Lewis acid, and the BL base, NH3, also fits the definition of the Lewis base.
However, the Lewis definition is broader and covers more situations. For the following reaction, B(CH3)3 is the Lewis acid because boron has an incomplete octet, and the empty 2p orbital on boron is able to accept electrons. (CH3)3N behaves as the Lewis base with the lone pair electron on N that is able to be donated.
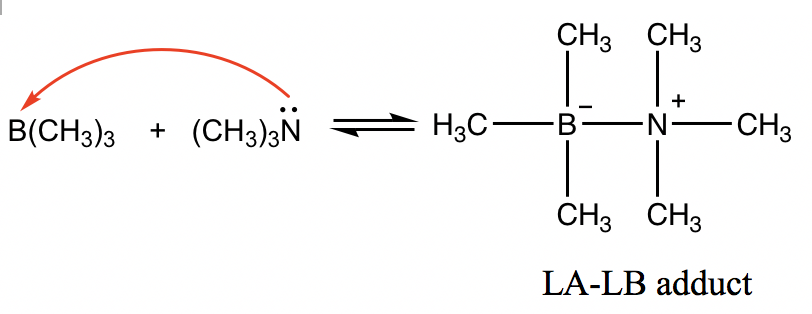
The product between Lewis acids and Lewis bases is usually a species that has the acid and base joined together, and this product is called the “LA-LB adduct”.
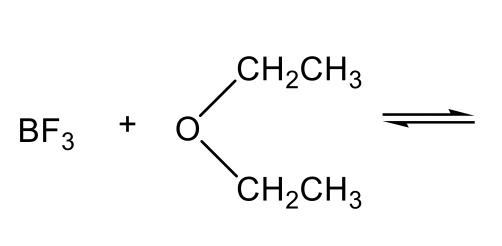
Other examples of Lewis acids include electron-deficient species, such as H+, M+, M2+, BH3, BF3, and AlCl3. Lewis bases can be amine, ether, or other species that have lone pair electrons to donate.
Exercises 3.3