Chapter 10: Alkenes and Alkynes
10.5 Reaction of Alkenes: Hydrogenation
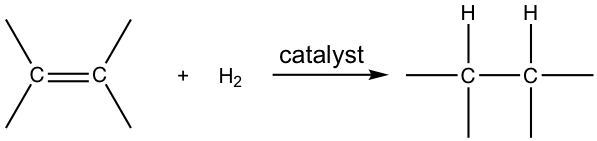
When alkenes react with hydrogen gas in the presence of a variety of metal catalysts, a hydrogen molecule will be added to the double bond in a way that each carbon atom bonds with one hydrogen atom. Such an addition reaction is called hydrogenation.
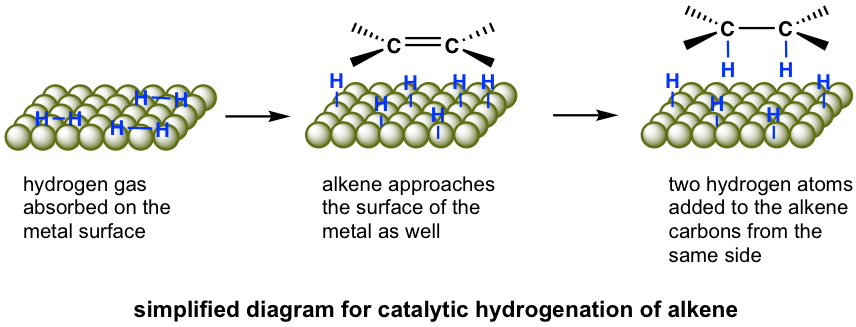
Catalysts are a must-have for hydrogenation, so the reaction can also be called catalytic hydrogenation. Commonly applied metal catalysts include palladium and platinum. Palladium, which is used as a powder absorbed on charcoal to maximize the surface area, is the most common catalyst and is referred to as palladium on charcoal (Pd/carbon). Platinum, which is usually used as oxide PtO2, is also employed frequently and referred to as Adams’ catalyst. These metal catalysts are not soluble in the reaction mixture and therefore are described as heterogeneous catalysts. The heterogeneous catalyst can be easily filtered out of the reaction mixture after the reaction and then recycled and reused.
![]()
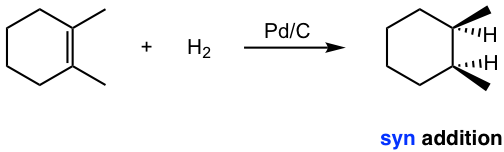
The hydrogenation reaction does not take place without a catalyst because of the enormous activation energy. The catalysts lower the activation energy by weakening the H-H bond and making the reaction feasible at room temperature. The details of the mechanism of catalytical hydrogenation are not completely clear. What was understood was that hydrogen gas is adsorbed on the surface of the metal, and the alkene also complexes with the metal by overlapping its π orbitals with vacant orbitals of the metal. The reaction occurs on the surface of the metal catalyst, with both hydrogen atoms added from the same side of the alkene to give alkane as the product that diffuses away from the metal surface. This mode of addition that the atoms added from the same side of the alkene is called the syn addition.

Example: