Chapter 1: Basic Concepts in Chemical Bonding and Organic Molecules
1.4 Resonance Structures in Organic Chemistry
The Resonance stabilization effect (also known as the resonance effect), as briefly mentioned in Section 1.3, is one of the fundamental concepts of Organic Chemistry and has broad applications. The discussion of the resonance effect heavily relies on the understanding of resonance structures. Here, we will focus on how to draw resonance structures (or resonance contributors) for organic chemistry species and how to compare the relative stabilities between the structures.
According to the resonance effect, the greater the number of resonance contributors, the greater the resonance stabilization effect, and the more stable the species is. Therefore, to predict whether the resonance effect applies or not, we usually need to construct “new” resonance structures (contributors) based on the “original” one available. Some very important rules need to be followed for such purposes.
Guidelines for Drawing Resonance Structures:
- All resonance structures must be valid Lewis structures. (Keep in mind that all the rules applied to Lewis structures still apply here!)
- All resonance structures must have the same atom connectivity and only differ in the electron arrangement. (Atoms NEVER move; only electrons move.)
- All resonance structures have the same number of electrons and net charge. (Formal charges on individual atoms could be different, but the net charge, which is the sum of all the charges, must be the same.)To move electrons, only π electrons and lone pair electrons (NEVER move σ bonds!) can be moved from a higher electron density area to a lower electron density area by following one of the three transformations:
-
- a π bond forms another π bond;
- a π bond forms the lone pair electrons; and
- lone pair electrons form a π bond.
- Use curved arrows to indicate the electron movement in the “original” resonance structure. The “new” resonance structure should be a “product” automatically obtained by following the arrows.
- Calculate the formal charge in the “new” structure and label any non-zero formal charges.
The way to use curved arrows to show electron transfer is also called arrow pushing, and it is a very important fundamental skill you need to master in organic chemistry. To construct “new” resonance structures, arrows have to be shown in the “original” structure.
Examples: Draw another resonance structure based on the given one.
1.
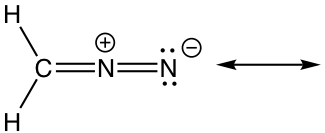
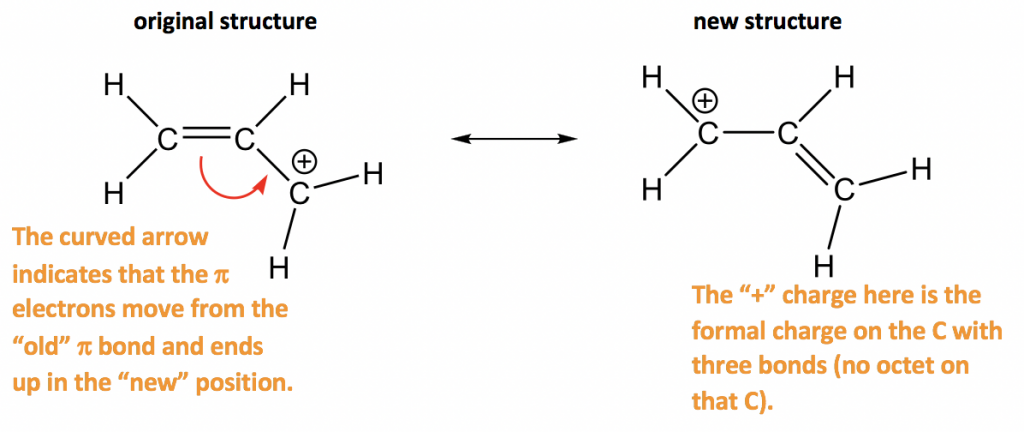
Approach: There is only one π bond in this example, and no any lone pairs, so only the π electrons can be moved around. There is a carbocation beside the π bond, which is the low electron density spot. Therefore it is reasonable to move the π electrons to the position beside carbocation to form another π bond, and that gives the “new” structure. The two resonance structures here are equivalent.
Solution:
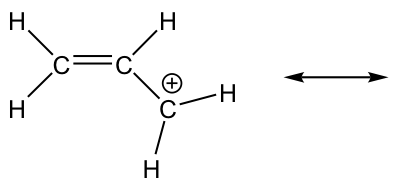
2.
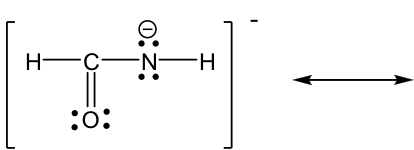
Approach: More electrons available for movement in this example: several lone pairs and one π bond. The guideline of “move electrons from the higher electron density area to the lower electron density area” provides a useful hint about where to start. The nitrogen atom has a “-” formal charge, meaning it has a relatively high electron density, higher than other neutral spots. So it is reasonable to move the lone pair on nitrogen away to form a π bond (keep in mind that lone pair can only form π bond, not another lone pair). However, when the new π bond is formed around the carbon atom, there are 5 bonds (10 electrons) on that carbon, which is not allowed. So, another electron pair has to be moved away, and the only available electron pair to be moved is the π electrons in C=O bond. It can be moved onto the oxygen atom and become another lone pair on the oxygen atom.
Solution:
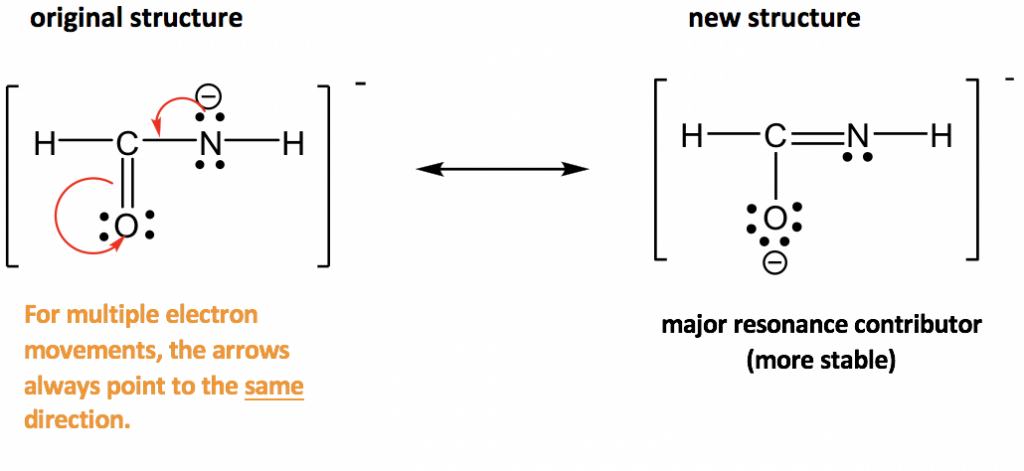
The two resonance structures in this example are non-equivalent, so one is more stable than the other. By applying the formal charge guideline, the “-“ formal charge is more preferable on oxygen, which is more electronegative than nitrogen, so the 2nd structure is the more stable one with lower energy, and makes more contribution to the actual structure in this species. The more stable structure can also be called as the major resonance contributor.
Comparing the relative stability of different resonance contributors:
- Structures with a maximum of octets are the most important.
- Charge separation usually decreases the stability (increases the energy of the contributor).
-
Negative charges should be preferentially located on atoms with greater electronegativity, and positive charges should be preferentially located on atoms with less electronegativity.
Common errors in drawing resonance structures:
- σ bond is moved.
- An Atom is moved.
- There are more than eight electrons located around C, N or O.
- Arrows are not shown in the proper way.
- Electron pairs are moved too far away; they should only be moved to the next position/atom.
Exercises 1.7 Draw new resonance structure and compare the relative stability, show arrows in the original structure.
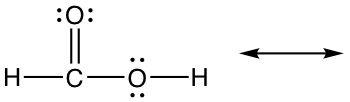
2.