Chapter 4: Conformations of Alkanes and Cycloalkanes
4.1 Conformation Analysis of Alkanes
4.1.1 Conformation
At a molecular level, a property of σ (sigma) bonds in alkane is that the bonds keep on rotating. For the example of ethane (CH3CH3), one methyl (CH3) group is able to rotate around the C-C bond freely without any obstacles.
The C-C bond is formed by the sp3-sp3 orbitals overlapping and the bond is cylindrically symmetrical, so rotation about the bond can occur easily and the molecule does not seem to change. However, a closer look indicates that the rotation of the C-C bond does result in a different spatial arrangement of hydrogen atoms in the molecule, as shown below:
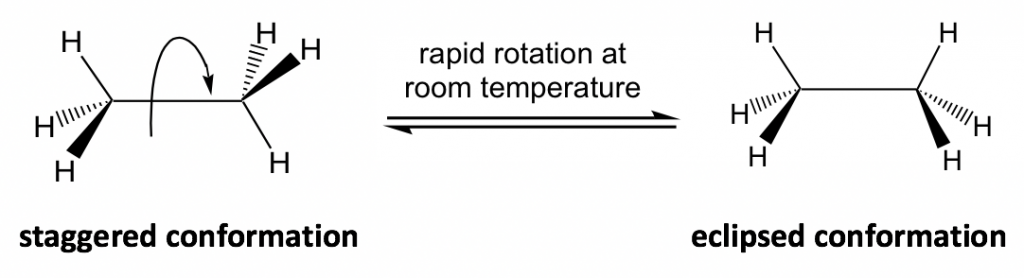
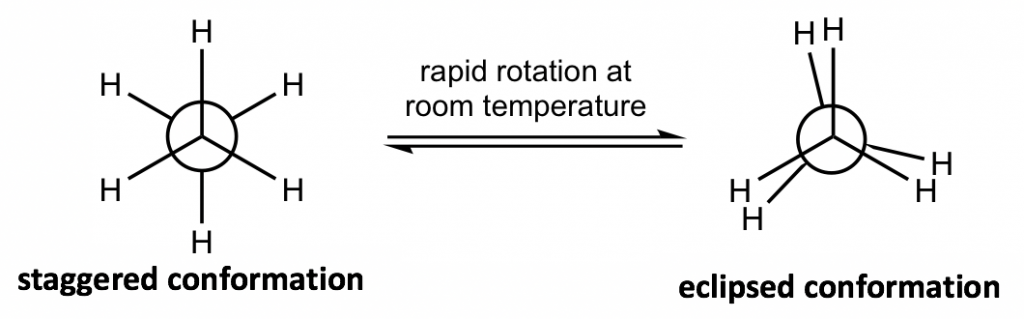
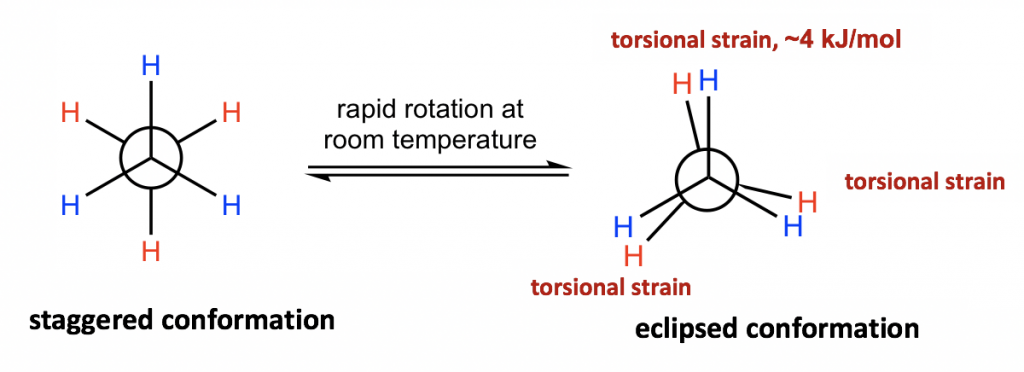
The different spatial arrangements of the atoms/groups that result from the single bond rotation are called conformations. Molecules with different conformations are called conformational isomers or conformers. The two extreme conformations of ethane coming from the C-C rotation shown above are: the staggered conformation with all of the H atoms spread out and the eclipsed conformation with all of the H atoms overlapped.
In the study of conformation, it is convenient to use certain types of structural formulas. The formula used in the drawing above is the perspective formula (see section 2.1.1) that shows the side-view of the molecule. In perspective formulas, solid and dashed wedges are used to show the spatial arrangement of atoms (or groups) around the sp3 carbons.
Another structural formula is the sawhorse formula which shows the tilted top-view of the molecule.
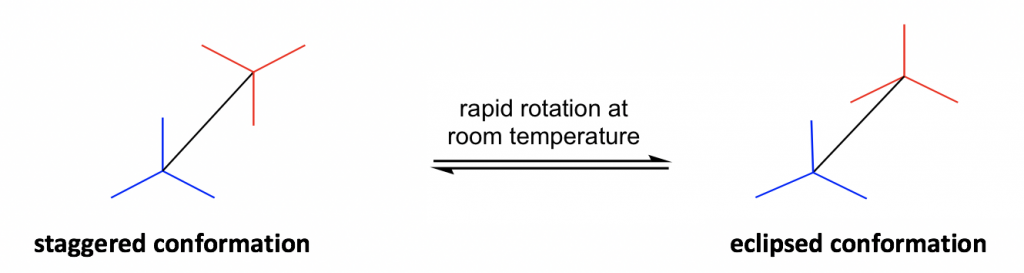
The most commonly applied formula in conformation analysis is the Newman projection formula.
How to draw a Newman projection
To draw a Newman projection, we will imagine viewing the molecule from one carbon to the next carbon atom directly along a selected C-C bond, as shown below, and follow the rules:
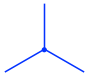
- The front carbon atom is shown as a point with three other bonds:
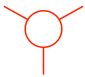
- The rear carbon atom is shown as a circle with three other bonds:
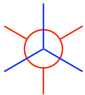
- Put the two carbons together to get the Newman projection of the staggered conformation:
- From the staggered conformation, fix the front carbon in place and rotate the rear carbon by 60° to get the eclipsed conformation:
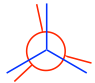
Note: In eclipsed conformers, the C-H bonds are supposed to be completely overlapped; however, to make the rear groups still visible, the bonds on the rear carbon are intentionally drawn slightly tilted.
4.1.2 Conformation Analysis of Ethane
Next, we will do a conformation analysis of ethane by using the Newman projections. A conformation analysis is an investigation of the energy differences and relative stabilities of the different conformations of a compound.
The two conformations of ethane, staggered and eclipsed, are different and therefore should be in different energy levels. You may also intuitively predict that the staggered conformation is more stable and has lower energy because the C-H bonds are arranged as far apart as possible in that conformation. That is correct! In eclipsed conformations, the H atoms on the front carbon overlap with the H atoms on the rear carbon, and this arrangement causes the repulsion between the electrons of the C-H bonds of the two carbons. This type of repulsion is called torsional strain, also known as eclipsing strain. Due to torsional strain, the eclipsed conformer is in an energy level that is 12 kJ/mol (or about 2.9 kcal/mol) higher than the staggered one. This can be represented graphically in a potential energy diagram as shown in Figure 4.1f.
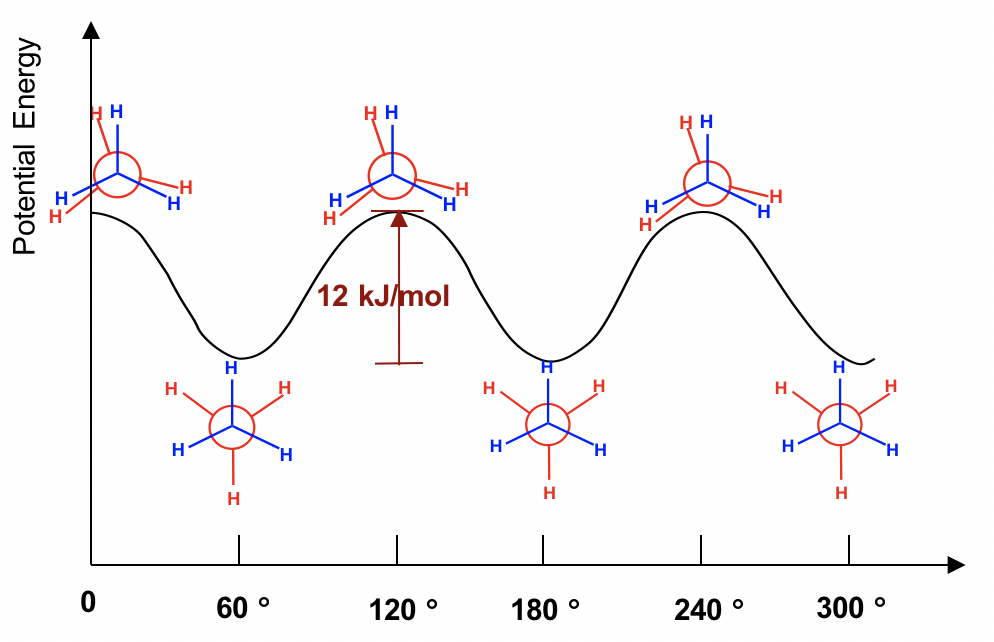
Because of this energy difference, an energy barrier must be overcome when rotation about the C-C bond occurs. However, this energy difference in ethane is small, and the kinetic energy of molecules at room temperature is high enough to cover it. So, at room temperature, the changes from staggered to eclipsed conformers occur millions of times per second. Because of these continuous interconversions, these two conformers cannot be separated from each other. However, at any given moment, about 99% of the ethane molecules will be in a staggered conformation because of their higher stability.
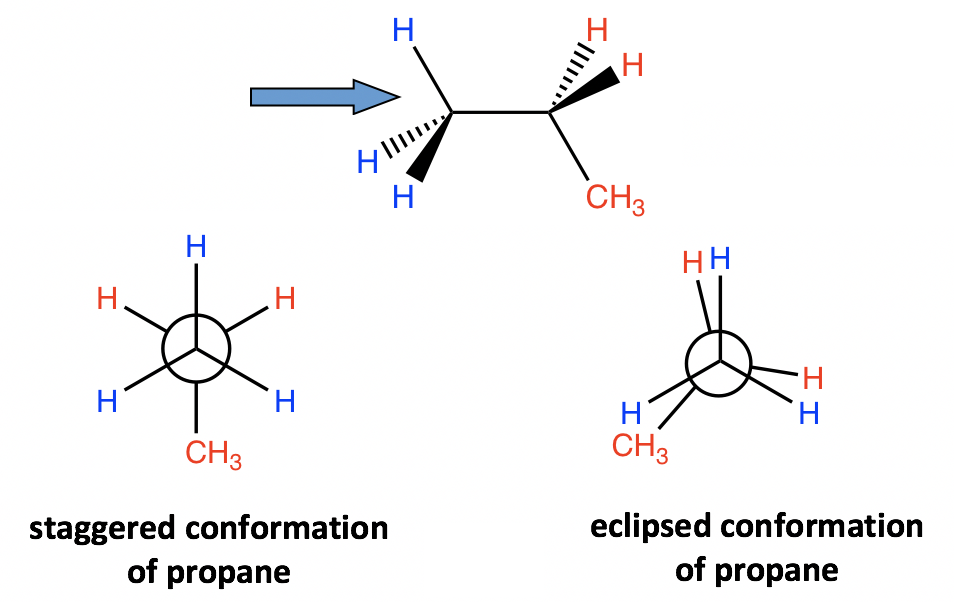
4.1.3 Conformation Analysis of Propane
A similar analysis can be applied to propane as well. There are still two types of conformations: staggered and eclipsed resulting from the rotation. The difference between propane and ethane is that there is a methyl (CH3) group connected to the rear carbon for propane. However, that does not affect the relative stability, and the staggered conformer is more stable and has lower energy.
4.1.4 Conformation Analysis of Butane
There are three C-C bonds in butane, and rotation can occur about each of them. If we choose C1-C2 (or C3-C4) for the study, the situation is almost the same as propane, with the ethyl CH2CH3 group replacing the CH3 group. However, if we consider the rotation along the C2-C3 bond, the situation will be much more complex.
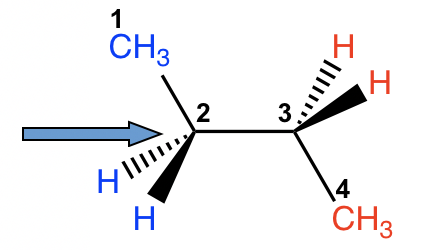
For both carbon atoms, C2 and C3, there are two hydrogen atoms and one methyl CH3 group bonded. We can start with the conformer in which the two CH3 groups are opposite to each other, then fix the front carbon and do 60° rotations of the rear carbon to investigate all the possible conformations.
Exercises 4.1: Draw all the possible conformers of butane from viewing along the C2-C3 bond. Finish this practice by yourself before continue reading!
Tips for drawing all the possible conformers about a certain C-C bond:
- View along that C-C bond; circle and decide what atoms/groups are connected on each carbon;
- Start with the staggered conformation in which the largest groups on each carbon are opposite (far away) to each other (this is called the “anti” conformation as we will learn later);
- Keep the groups on one carbon “fixed”, and rotate the groups on the other carbon at 60° angles. Repeat the rotation five times, and you should get a total of six conformers.
Answers to Chapter 4 Practice Questions
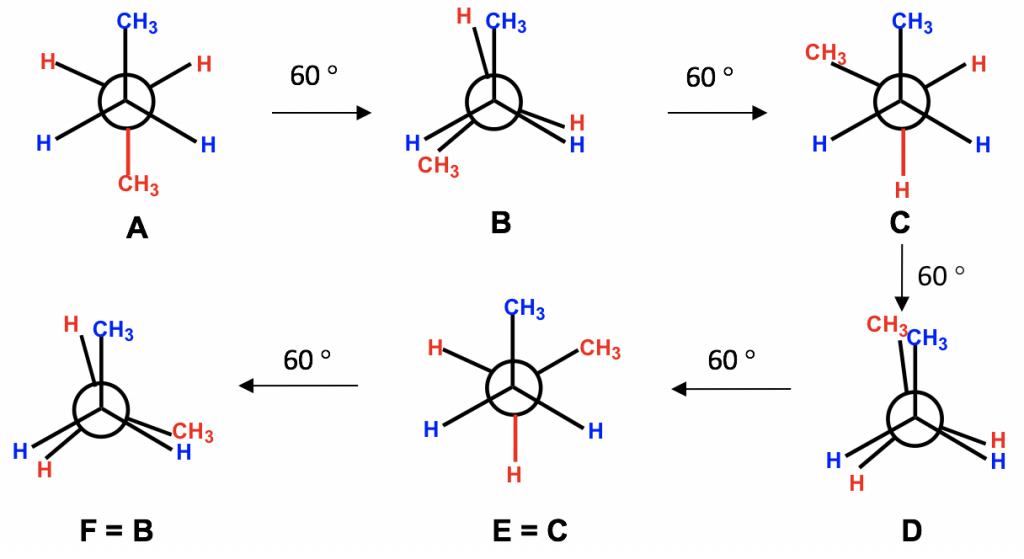
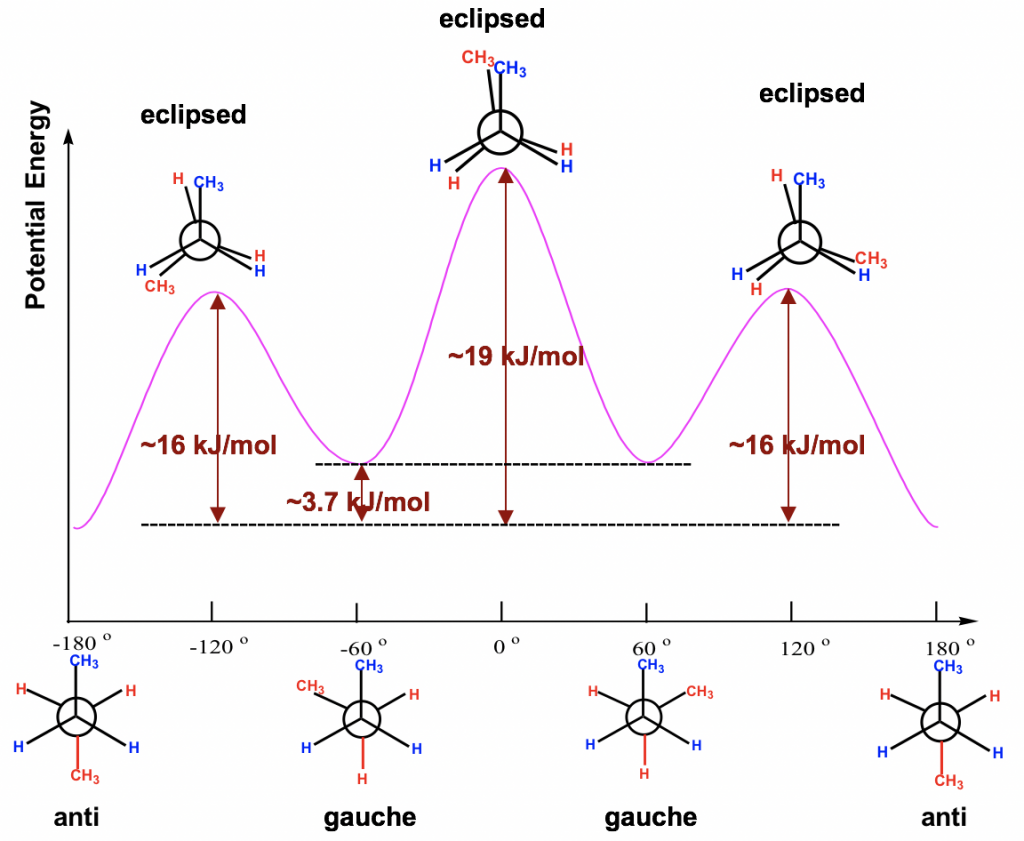
Among all six conformers obtained, there are three staggered and three eclipsed. Staggered conformations C and E should be in the same energy level because the groups are arranged in an equivalent way between these two conformers. Similarly, eclipsed conformations F and B are also in the same energy level. So, our studies can be focused on the four conformers: A, B, C and D, which are different in terms of energy and stability.
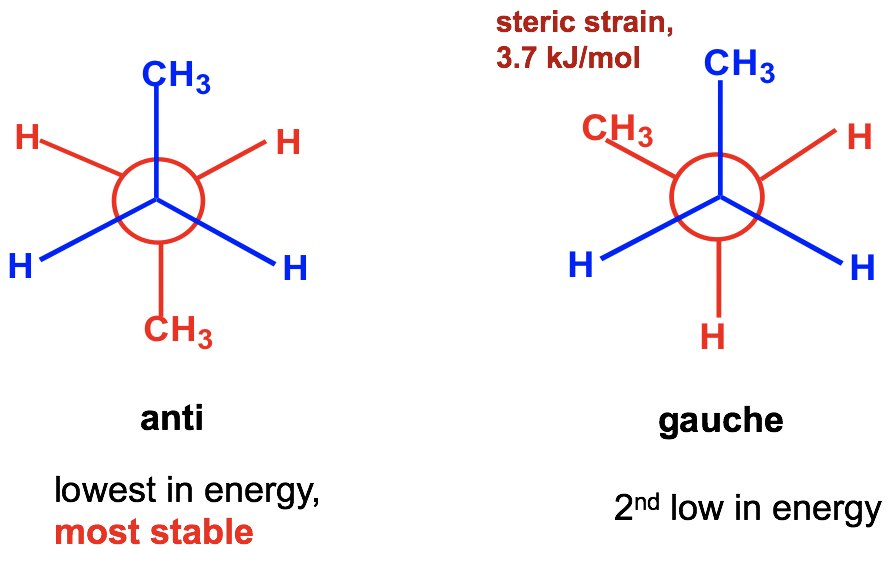
Between the two staggered conformers A and C, A is more stable than C because the two methyl CH3 groups in A are as far apart as possible. This most stable staggered conformation is called the anti-conformation (anti is Greek for “opposite”). In anti-conformations, the largest groups on the front and rear carbon are 180° opposite to each other. The other staggered conformation C is called a gauche conformation, in which the two large groups are adjacent and are 60° to each other. With the large groups being close to each other in gauche conformers, the molecule experiences steric strain. Steric strain is the strain that is caused when atoms (or groups) are close enough together that their electron clouds repel each other. Steric strain only matters when the groups are close to each other (less or equal to 60°), so steric strain does not apply in anti-conformations. The magnitude of steric strain also depends on the size of the group: the larger the size, the higher the steric strain. As a result, there is no steric strain between two small hydrogen atoms, even if they are close to each other.
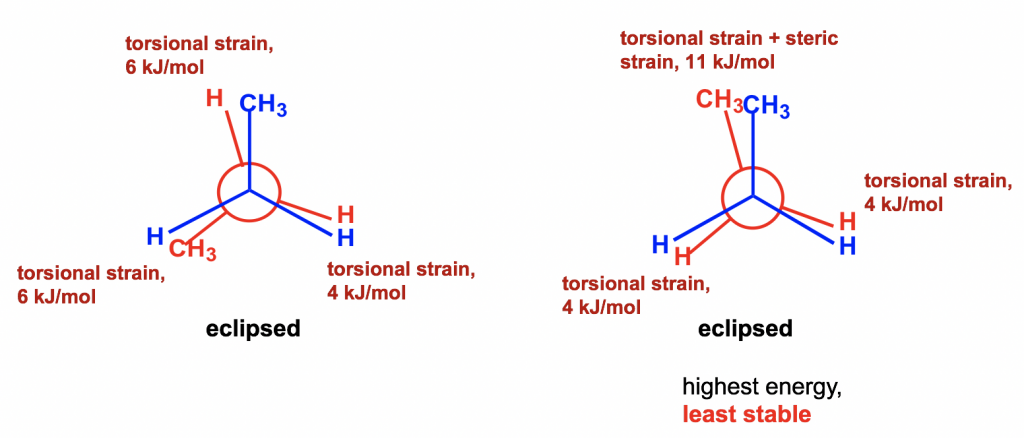
Between the two eclipsed conformers B and D, D is less stable than B because the two CH3 groups are eclipsing (overlapping) each other in D, causing both torsional and steric strains.
The energy difference of all the conformers obtained from the rotation about the C2-C3 bond are shown in the potential energy diagram Fig. 4.1l. The curve is more complex than that of ethane since there are four different energy levels corresponding to four conformers with different stabilities. Even though the energy barriers for the rotations are larger than that of ethane, they are still not high enough to stop rotation at room temperature.