Chapter 8: Elimination Reactions
8.4 Comparison and Competition Between SN1, SN2, E1 and E2
It is important to understand that the structural nature of a substrate (primary, secondary, or tertiary) is the most critical factor in determining which reaction pathway it goes through. For example, primary substrates never go with SN1 or E1 because the primary carbocations are too unstable. If the substrate could go with a couple of different reaction pathways, then the reaction conditions, including the basicity/nucleophilicity of the reagent, temperature, solvent, etc., play an important role in determining which pathway is the major one. Our discussions therefore will start with the different types of substrates, then explore the condition effects on that substrate.
Methyl
This is the easiest case. A methyl substrate only goes with SN2 reaction, if any reaction occurs. Elimination is not possible for methyl substrates, and no SN1 reaction is possible either because CH3+ is too unreactive to be formed, so the only possible way is SN2.
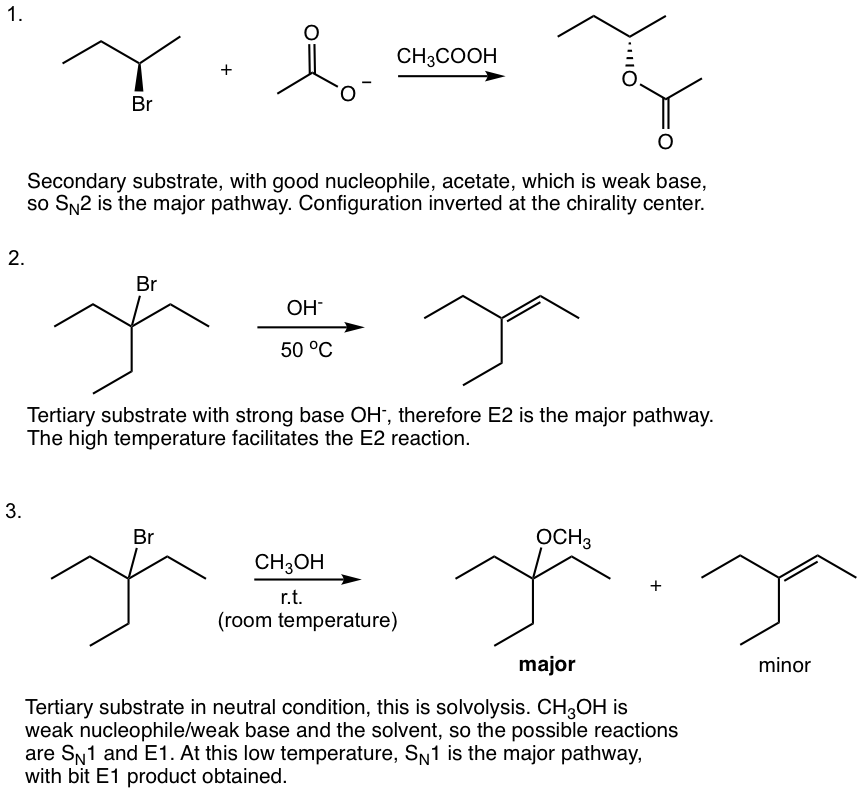
Primary (1°)
Primary (1°) substrates cannot go with any unimolecular reaction, that is no SN1/E1, because primary carbocations are too unstable to be formed. Since primary substrates are very good candidates for SN2 reactions, SN2 is the predominant pathway when a good nucleophile is used. The only exception is that when a big bulky base/nucleophile is used, E2 becomes the major reaction.
Examples of reactions for primary substrates:
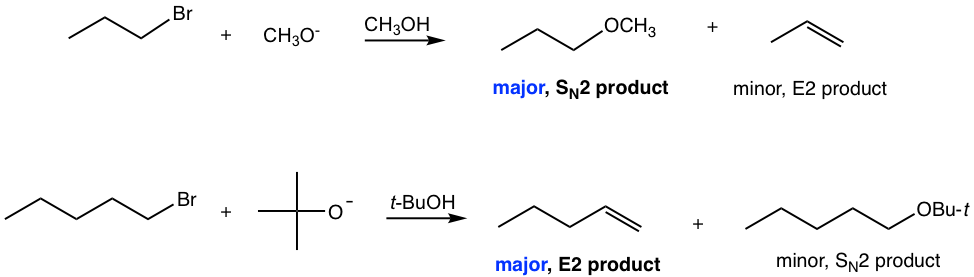
Secondary (2°)
It is most complicated or challenging to predict the reaction of a secondary substrate (2°) because all the pathways are possible. The reaction conditions then become a key factor. The four types of reactions can be separated into three pathways:
E2: favored by a strong base
SN2: favored by a good nucleophile (relatively weaker base)
SN1/E1: It is hard to separate SN1 and E1 completely because they both go through carbocation intermediates and are favored by a poor nucleophile/weak base, for example, H2O or ROH (solvolysis). Under such neutral conditions, SN1 and E1 usually occur together for secondary substrates, and increasing the reaction temperature favors E1 over SN1.
It is relatively easy to separate SN2 and E2 pathways from SN1/E1 since both SN2 and E2 require a strong nucleophile or strong base, which are usually negatively charged species, while SN1/E1 requires neutral conditions.
To distinguish SN2 from E2, we need to determine whether a negatively charged anion is a strong nucleophile (for SN2) or a strong base (for E2). All nucleophiles are potential bases, and all bases are potential nucleophiles because the reactive part of both the nucleophile and base are lone pair electrons. Whether an anion is a better nucleophile or a better base depends on its basicity, size, and polarizability. Generally speaking, the relatively stronger bases have the tendency to act as bases, and relatively weaker bases with a small size and good polarizability have the tendency to act as nucleophiles. See the list given below.
Strong bases: OH–, RO–(R: small size alkyl group), NH2–
Good nucleophiles (relatively weaker bases): Cl–, Br–, I–, RS–, N3–, CN–, RCO2–, RNH2
Please note that bulky bases, such as t-BuO– and LDA, always favor E2 and generate elimination products that follow Hofmann’s rule because they are too big to do a back-side attack in SN2.
Examples of reactions for secondary substrates:

Tertiary (3°)
Tertiary (3°) substrates do not go with SN2 reactions because of steric hindrance. So an E2 reaction is the choice when a strong base is applied or an SN1/E1 pathway with neutral conditions (poor nucleophile/weak base). Theoretically speaking, E2 and E1 are supposed to give the same elimination product. However, to synthesize an alkene from a tertiary substrate, it is a better choice to use a strong base that encourages the E2 process rather than E1. This is because E1 always combines together with SN1, and it is almost impossible to avoid the substitution product.
The above discussions can be briefly summarized in Table 8.2 below, followed by several examples. To predict the reaction outcome or to design a synthesis route for a certain case, it is highly recommended that you do the analysis by following the logic mentioned above, instead of just referring to the table. Also, practice makes perfect!
|
Substrate |
Preferred Reaction Pathways |
|---|---|
|
Methyl |
SN2 reaction |
|
Primary |
Predominantly SN2 reaction;
Exception: E2 reaction for bulky base |
|
Secondary
|
SN2 reaction with a good nucleophile (e.g., RS–, RCO2–, etc)
E2 reaction with a strong base (e.g., OH–, OR–) SN1/E1 with neutral condition (e.g., H2O, ROH) |
| Tertiary | E2 reaction with a strong base (e.g., OH–, OR–)
SN1/E1 with neutral condition (e.g., H2O, ROH) |
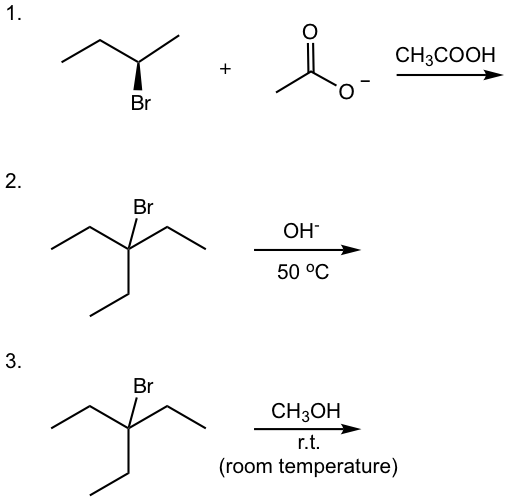
Examples: Show major organic product(s) for following reactions.
Solutions: