Chapter 1: Alcohols and Ethers
1.1 Structures, Naming and Physical Properties
1.1.1 Alcohols
Alcohol is a type of compound that contains one or more hydroxyl hydroxy (OH) groups bonded to a saturated carbon atom (or sp3 hybridized carbon). The most common alcohol in our daily life is ethanol, with the formula of C2H5OH, which is the active ingredient in all alcoholic beverages. Fermentation of the sugars from a variety of sources, for example, fruit juices, and grains, with the help of yeast produces ethanol. Methanol, the smallest alcohol, was used to be produced by heating wood at a high temperature in the absence of air, therefore has another name “wood alcohol”. As an important industrial chemical today, most methanol is produced by the catalytical hydrogenation of carbon monoxide with high pressure and high temperature.

The nomenclature rules we learned in section 2.4 in Book I, cover the IUPAC naming of simple alcohol compounds (as the examples given in Fig. 1.1.a). Alcohols could also be classified as 1° (primary), 2° (secondary), or 3° (tertiary), depending on the number of carbons bonded to the alcohol carbon, as shown in the above examples.
Compounds that have an OH group connected directly to a benzene ring are called phenols. Phenol is a type of aromatic alcohol.
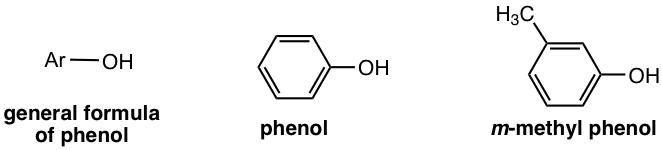
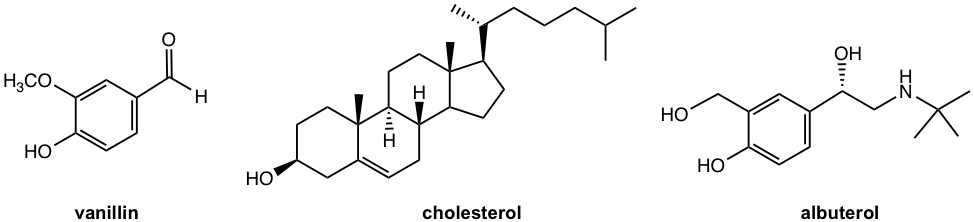
The alcohol group may also be part of a more complex molecule, for example in vanillin and cholesterol. Some larger molecules can contain multiple OH groups, such as in albuterol, the chemical ingredient used in some respiratory medicines for the treatment of difficulty breathing.
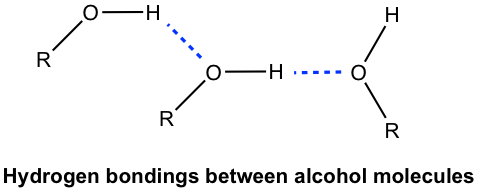
The physical properties of alcohols, such as boiling points and solubilities, are affected significantly by the presence of the OH group. The boiling point of alcohols is much higher than alkanes with the same number of carbons, due to the hydrogen bonding of the OH group. For example, the boiling point of ethanol is 78°C while ethane has the boiling point of –88°C.
The high polarity of the OH group also contributes to the good solubility of smaller alcohols (up to butanol) in water. As the length of the carbon chain increases, the solubility of alcohol decreases since the size of the hydrophobic potion overrides the hydrophilic potion (refer to section 2.6 in Book I).
|
Name |
Structure |
Boiling Point (°C) |
Solubility in water at 23 °C |
|
Methanol |
CH3OH | 65.0 | infinite |
| Ethanol | CH3CH2OH | 78.5 | infinite |
| 1-Propanol | CH3CH2CH2OH | 97.4 | infinite |
| 1-Butanol | CH3CH2CH2CH2OH | 117.3 | 8.0g / 100mL |
| 1-Pentanol | CH3CH2CH2CH2CH2OH | 138 | 2.2g / 100mL |
Table 1.1 Physical properties of some common alcohols
1.1.2 Ethers
Ether is a type of compound that contains an oxygen atom bonded to two carbon atoms. The hydrocarbon groups may be alkyl, alkenyl, vinyl, or aryl. Other than open chain structure, ether may also be in ring structure. Several examples of ethers are shown below. Diethyl ether is an organic solvent and was at one time widely used as an anesthetic agent but was then replaced by other substances due to its slow effect and recovery time, plus its high inflammability. THF is the common polar aprotic solvent, as we learned in section 7.5 in Book I.
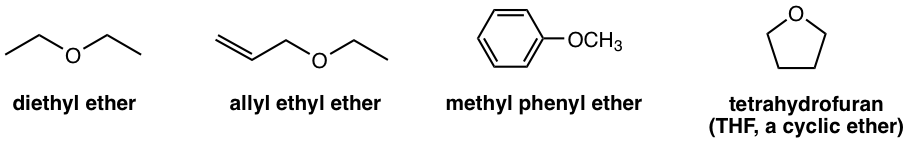
The naming of the ether can be referred to in section 2.4 in Book I. Simple ethers are named as common name “alkyl alkyl ether” with the alphabetic order of the alkyl groups. For the structure with other groups with higher priorities, ether is regarded as the substituent and named with the “oxy” prefix.
The boiling points of ethers are roughly comparable with those of hydrocarbons of similar molar mass, since the ether group is just slightly polar. As an example, diethyl ether (MM = 74 g/mol) has a boiling point of 34.6 ºC, while pentane (MM = 72 g/mol) has that of 36 ºC.

