Chapter 2: Aldehydes and Ketones
2.2 Preparations of Aldehydes and Ketones
In the early chapter and Book I, we have learned several reactions that provide good methods for the preparation of aldehydes and/or ketones, let’s have a review here.
Chapter 1 Review
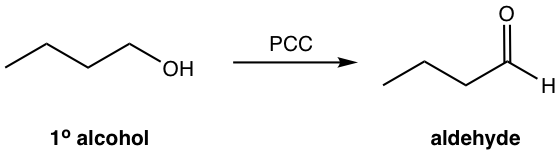
Oxidation of 1° or 2° Alcohols (1.2.5)
Pyridinium chlorochromate (PCC) is the proper oxidizing reagent to oxidize 1° alcohol to aldehyde, and oxidize 2° alcohol to ketone.
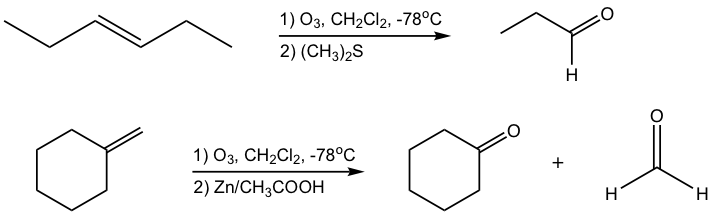
Ozonolysis of an Alkene (10.7.2 in Book I)
Ozonolysis reaction is the oxidative cleavage of the C=C double bond in the alkene, that produces aldehyde and/or ketone as products. It is a two-step process in which alkene reacts with ozone at a very low temperature (-78 °C) first, then treated with dimethyl sulfide, (CH3)2S, (or Zn/CH3COOH) to give the products. A couple of examples are shown here.
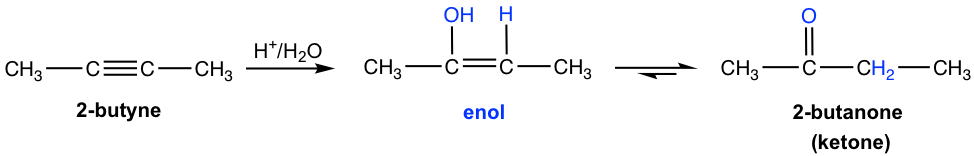
Hydration of an Alkyne (10.8 in Book I)
Starting from terminal alkyne, aldehyde can be prepared by hydroboration-oxidation, and methyl ketone can be prepared by hydration with Hg2+ as a catalyst.
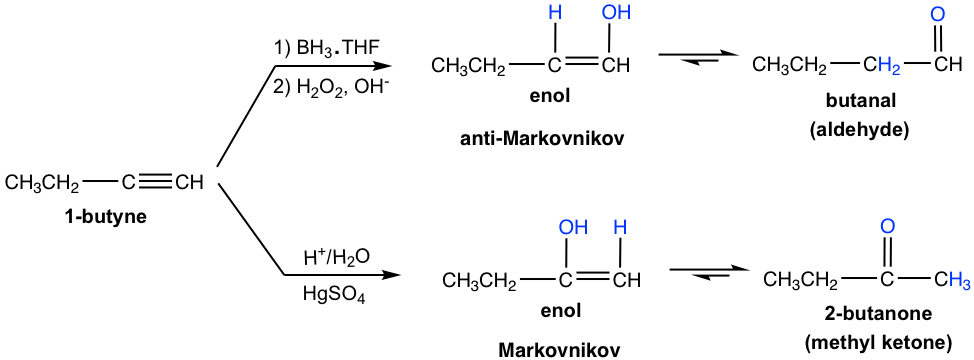
For internal alkyne, hydration with Hg2+ as a catalyst generates ketone as a product. For synthesis purposes, this method works best with symmetric internal alkyne that gives only one ketone product.
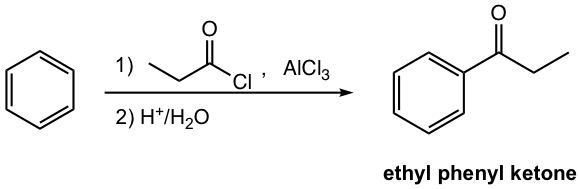
Friedel-Crafts Acylation
Another important reaction that gives ketone as a product is Friedel-Crafts acylation, a type of Electrophilic Aromatic Substitution (EAS) reaction. We will have thorough discussions on EAS and Friedel-Crafts reaction in Chapters 4 and 5. An example is shown here for general purposes.