Chapter 7: Carboxylic Acid and Derivatives
7.3 Reactions of Carboxylic Acid and Derivatives: Nucleophilic Acyl Substitution
7.3.1 Two Categories of Carbonyl Compounds
The reactivity of carboxylic acid and derivatives still focuses on the carbonyl group, specifically on the electrophilic carbon because of the polarity of the C=O bond. The carbonyl group together with the R (or Ar) group attached is called the acyl group.
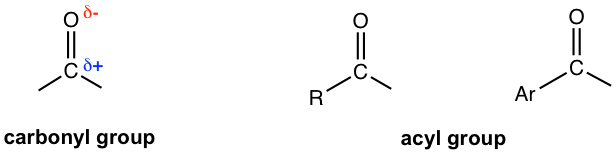
The above structures might remind you of aldehydes and ketones, which also contain the acyl group. Aldehydes and ketones, and carboxylic acids and derivatives are all carbonyl compounds since they all have the carbonyl group. However, they belong to two categories in terms of reactivity because the other group that is attached to the acyl group strongly affects the reactivity of the carbonyl compounds (Fig. 7.3b).

In aldehydes and ketones, the acyl group is attached to hydrogen or alkyl group (H or R) that are poor leaving groups and cannot be replaced by another group. In carboxylic acids and derivatives, the acyl group is attached to a group that contains the electronegative atom (OH, OR, Cl, NH2, etc), these groups are good (or reasonably good) leaving groups and can be replaced by another group. As a result, (nucleophilic) addition is the common reaction for aldehydes and ketones (Chapter 2), but carboxylic acids and derivatives usually undergo (nucleophilic)substitution instead.
7.3.2 General Mechanism of Nucleophilic Acyl Substitution
The substitution reaction of carboxylic acids and derivatives is generally called acyl substitution because it takes place on the acyl group. It is not a one-step substitution though, but goes through the addition-elimination mechanism.
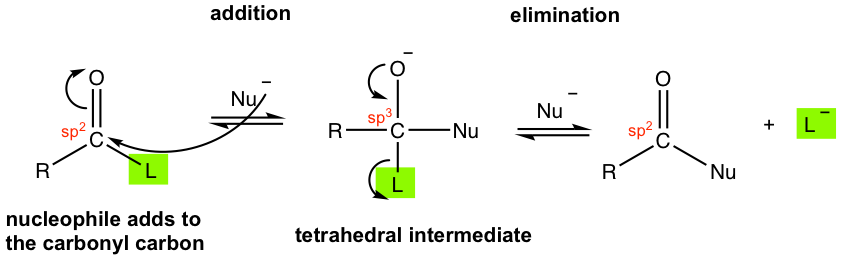
The mechanism in Fig. 7.3c represents the acyl substitution process with a negatively charged nucleophile. (If a neutral nucleophile reacts, there will be extra proton transfer steps, refer to section 7.5). In the above mechanism, the nucleophile first attacks the carbonyl carbon that bears the partial positive charge, and forms an intermediate. The intermediate is called a tetrahedral intermediate because the central carbon is the sp3 hybridized carbon in a tetrahedral shape. In the tetrahedral intermediate, both group L and the nucleophile contain electronegative atoms, for example, O or N, and therefore are very unstable. The group L leaves in the second step and another π bond is reformed to give the final product, in which the nucleophile replaces the group L.
Both steps in the above mechanism are reversible, which means the reaction has the chance to go backward as well. Whether the forward or the backward reaction is predominant in the equilibrium depends on the relative basicity (or leaving ability) between group L and the nucleophile. As we learned before in the SN1/SN2 reaction (section 7.3 in Book I), the weaker the base the better it is as a leaving group. Therefore, if L– is a weaker base than Nu–, i.e. L– is a better leaving group than Nu-, the reaction proceeds in the forward direction and produces the desired substitution product. On the other side, if Nu– is a weaker base (or a better leaving group) than L–, then the backward reaction predominates and it is not productive to carry out the reaction.
7.3.3 Relative Reactivity of Carboxylic Acid and Derivatives
Different carboxylic acid derivative has different reactivity towards acyl substitution reaction. In general, the order of reactivity is:
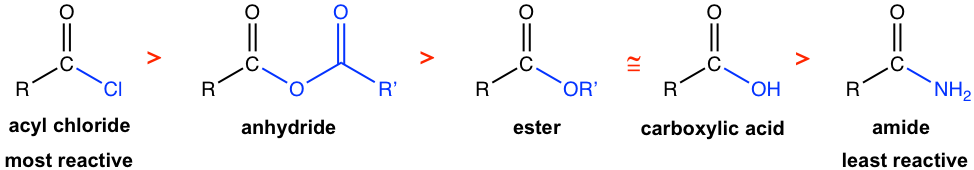
Acyl chlorides are the most reactive, and amides are the least reactive towards acyl substitution. As was discussed in the previous section the better the leaving group L, the more easily the acyl substitution takes place. So the relative reactivity of carboxylic acid derivatives could be rationalized by the leaving group ability of the group L, or the basicity of L–, in different derivatives. The weaker the basicity of L–, the better the leaving group is, and the more reactive the carboxylic acid derivative. The order of the basicity of leaving groups is shown below which matches the order of reactivity.
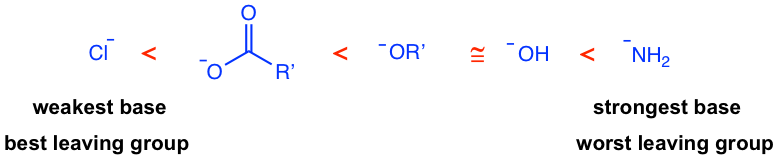
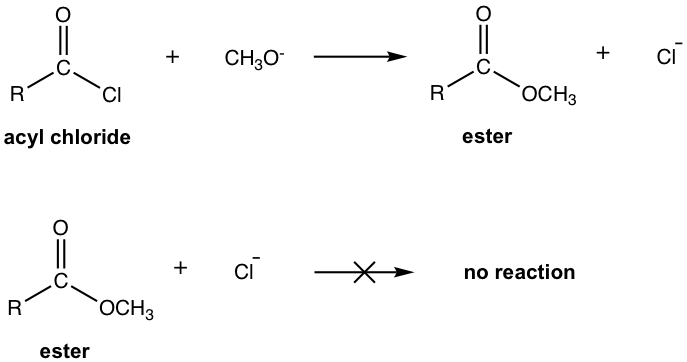
An important conclusion that arises from the relative reactivity listed above is that a more reactive carboxylic acid derivative can be converted into a less reactive derivative in a nucleophilic acyl substitution, but the reverse process is usually more difficult and requires special reagents when possible. This is because in the acyl substitution, the leaving group has to be a weaker base than the nucleophile. As a result, the most reactive acyl chloride can be used as a reactant to produce any of the other derivatives. For example, acyl chloride can be converted to an ester (Cl– is a weaker base than RO–), but the ester cannot be converted into any acyl chloride. The application of this trend in the synthesis of carboxylic acid derivatives is that the less reactive derivative can be prepared from the more reactive derivative, but not the other way.
Further discussions on the properties and reactions of each functional group are in the following sections.

