Chapter 7: Carboxylic Acid and Derivatives
7.1 Nomenclatures and Physical Properties of Carboxylic Acid Derivatives
In IUPAC nomenclature, a certain suffix is used to indicate a specific functional group as the parent structure of the compound. Two versions of suffixes of each functional group of carboxylic acid or a derivative are listed in Table 7.1. When the functional group is part of the parent carbon chain, the compound is named by replacing the terminal “e” of the hydrocarbon name with the first suffix for that functional group. If the functional group is attached to a ring structure, the second suffix is used by following the name of the ring. Specific examples are given in the table as well for illustration.
| Functional group |
Suffix |
Examples |
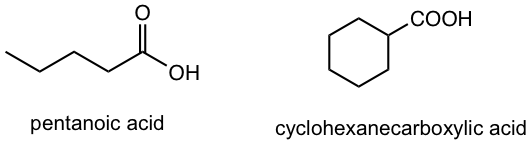
| acid | – oic acid
– carboxylic acid |
 |
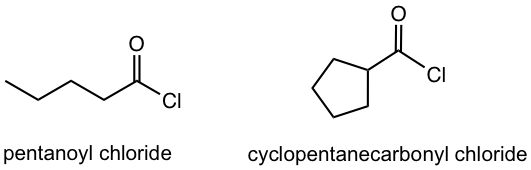
| Acyl halide | – oyl chloride
– carbonyl halide |
 |
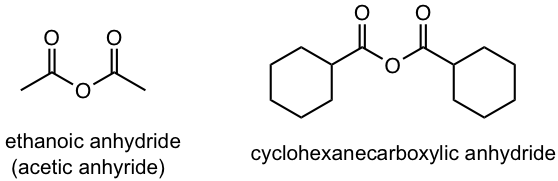
| anhydride | – oic anhydride
– carboxylic anhydrate |
 |
| ester | – oate
– carboxylate |
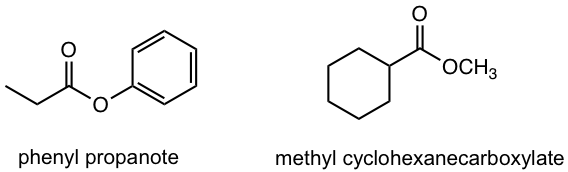 |
| amide | – amide
– carboxamide |
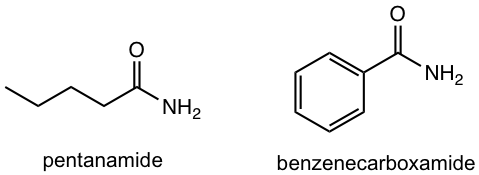 |
| nitrile | – nitrile
– carbonitrile |
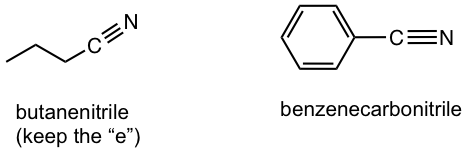 |
Table 7.1 Carboxylic acid derivatives suffix and examples
There are several extra notes about the naming of carboxylic acid derivatives, with an example given for each case.
Name of Ester
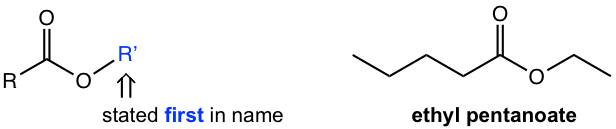 For the naming of an ester, the name of the alkyl group attached to the carboxyl oxygen is stated first, followed by the name of the acid with the proper suffix.
For the naming of an ester, the name of the alkyl group attached to the carboxyl oxygen is stated first, followed by the name of the acid with the proper suffix.
Name of Salts
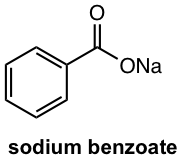
Salts of carboxylic acids are named with the same suffix as ester, that is “oate”, with the name of the metal put first.
Name of Anhydride, symmetric and mixed
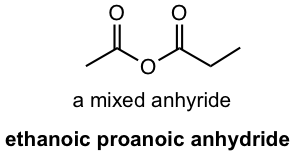 An acid anhydride is the functional group generated by the loss of water from two carboxylic acid molecules (section ?? for details). If the two acids are the same, the anhydride is symmetric. If they are different, the anhydride is a mixed anhydride. The mixed anhydride is named by starting the name of both acids in alphabetical order, followed by “anhydride”.
An acid anhydride is the functional group generated by the loss of water from two carboxylic acid molecules (section ?? for details). If the two acids are the same, the anhydride is symmetric. If they are different, the anhydride is a mixed anhydride. The mixed anhydride is named by starting the name of both acids in alphabetical order, followed by “anhydride”.
Amide with substituents on Nitrogen
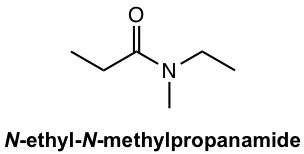
For amide, if a substituent is bonded to the nitrogen, the name of the substituent is stated first, preceded by a capital N, then followed by the name of the amide.
Properties of Carboxylic Acids and their Derivatives
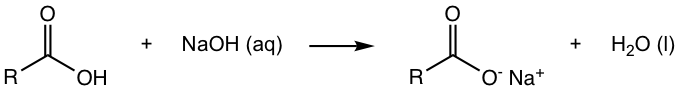
Carboxylic acid is a type of organic acid with pKa in the range of about 5 (section 3.3 in Book I). Carboxylic acid reacts with the solution of sodium hydroxide readily to form the sodium salt.
The presence of the electron-withdrawing group increases the acidity of carboxylic acid (section 3.4.3 in Book I) since those groups help to delocalize the negative charge of the conjugate base and make the conjugate base more stable.
The boiling point of carboxylic acid is generally much higher than that of ester or acyl chloride with a similar molar mass, because of the hydrogen bonds between each other (Fig. 7.1b).
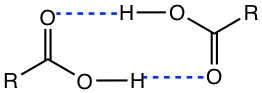
The carboxylic acid can also form hydrogen bonds with water, therefore the low-molecular mass carboxylic acids (up to four carbons) show appreciable solubility in water. Water solubility decreases with the length of the carbon chain increases.
Other Common Carboxylic Acids and Derivatives
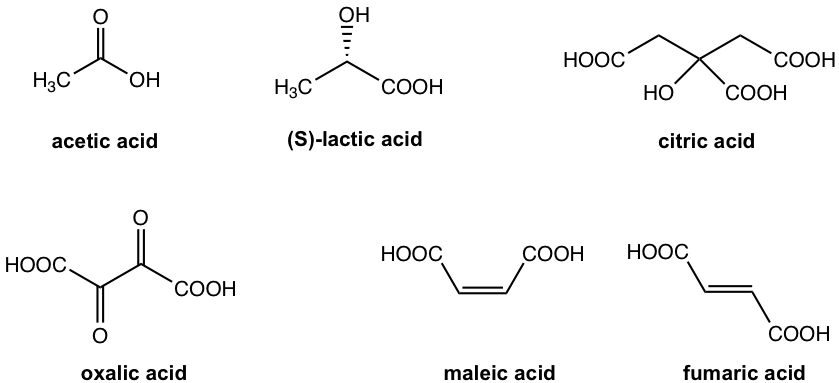
Carboxylic acids and esters are found widely in nature. The most well-known one is acetic acid, the ingredient in vinegar. (S)-Lactic acid is the compound responsible for the burning feeling of muscle after work out. Citric acid occurs naturally in citrus fruit, for example, lemons have the highest concentration and oranges have less. Some green leafy vegetables, such as spinach, are rich in oxalic acid. Maleic acid (Z isomer) and fumaric acid (E isomer) are the geometric isomers of butenedioic acid, that are both naturally present in honey. The structures of these common acids are shown in Fig. 7.1c.
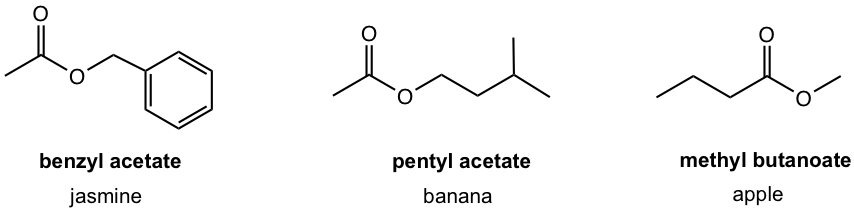
The pleasant odors of many flowers and fruits are due to esters, as shown in Fig. 7.1d. Without hydrogen atoms bonded to oxygen, esters cannot form hydrogen bonds, therefore the boiling points of esters are lower than that of acids with comparable molar mass.
Amide is the key functional group that forms the backbone of peptides and proteins.
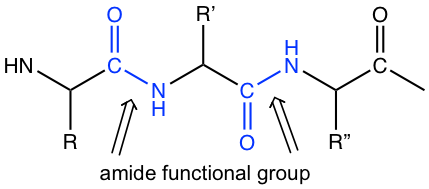
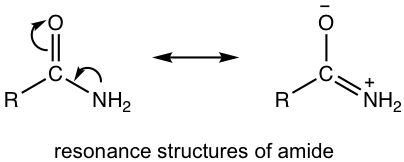
For the structure of amide, the resonance structure with charge separation makes a greater contribution to the hybrid than other derivatives, because the nitrogen atom can better accommodate a positive charge. Because of this reason, dipole-dipole force applies to amide molecules, which explains the high boiling points of amides. In addition, hydrogen bonds form for amides with NH or NH2 group, which also contributes to the high boiling points.