Chapter 6: Reaction at the α-Carbon of Carbonyl Compounds
6.1 The Acidity of the α-Hydrogens
Generally, the hydrogen atoms attached to sp3 carbons, for example in alkanes, are rather weak acids with pKa over 50. However, the α-hydrogens in the carbonyl compound, which also bond to the sp3 carbon, are much more acidic with pKa in the range of 16 to 20 (refer to section 3.3 in Book I for more discussions about pKa of different functional groups).
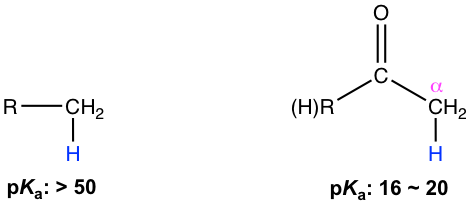
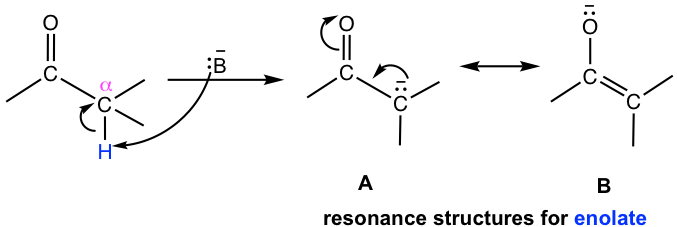
The presence of the carbonyl group is the reason behind the unusual acidity of the α hydrogens. For the anion that is produced after the loss of α hydrogen, the negative charge is delocalized to the carbonyl group via resonance effect, therefore the anion is stabilized significantly. The anion (or the conjugate base on the carbonyl compound) is called enolate. Both resonance structures A and B can be written for the structure of enolate, and the structure B makes more contributions to the hybrid because the negative charge is on oxygen, the atom with higher electronegativity.
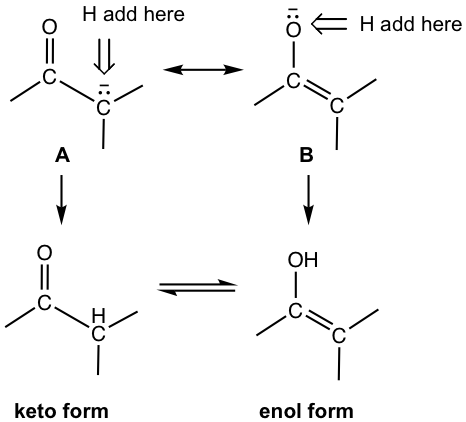
Keto and Enol Tautomers
When enolate accepts a proton, the protonation could occur on either carbon or oxygen and gives the two forms of the carbonyl compound, keto form and enol form respectively. The term “enol” means alkene + alcohol. The two forms are called tautomers, a special type of constitutional isomer (refer to section 10.8.3 in Book I). Keto and enol tautomers differ in the location of the double bond and hydrogen.
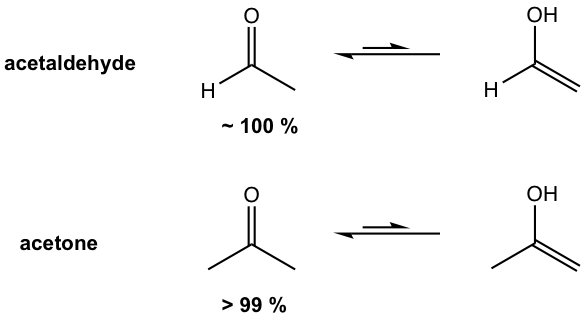
Tautomers are isomers that are in rapid equilibrium, and the interconversion between different tautomers is called tautomerization. For most aldehydes and ketones, the keto form is much more stable than the enol tautomer, and is predominant in the equilibrium.
An example of the special case in which enol form is the major species applies to β-diketones (more discussions in section 6.5). The amount of enol present at equilibrium is much higher than keto because enol is stabilized by two effects: the intramolecular hydrogen bonding and the conjugated double bonds.
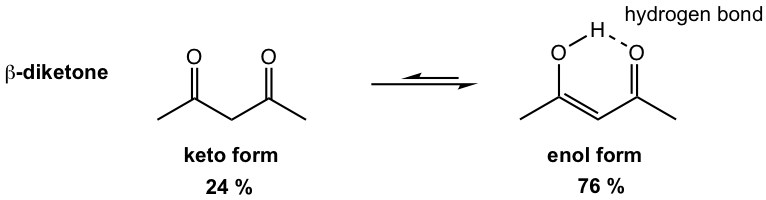
Keto and Enol Tautomerization
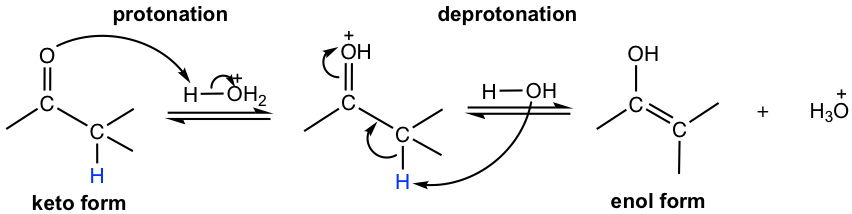
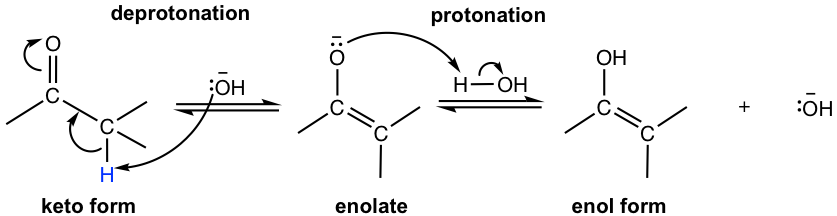
The tautomerization between keto and enol can be catalyzed by either an acid or a base, with the mechanism shown below.
In acidic conditions (Fig. 6.1f), the process is initiated by the protonation of the double bond, then followed by deprotonation of the α hydrogen.
Under basic conditions (Fig. 6.1g), the mechanism goes with the opposite order in which the α hydrogen is deprotonated first, then protonation of the enolate gives the enol form compound.
Examples
When a solution of the following chiral sample is treated with acid (or base), the solution gradually loses its optical activity. Explain the reason for this observation and show the reaction mechanism.
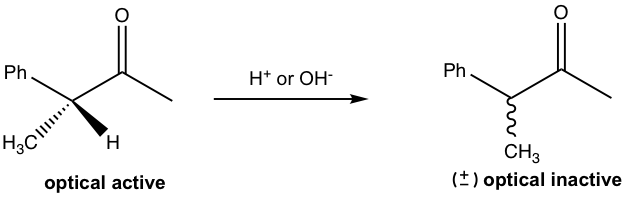
Answers: The mechanism for the reaction in acidic conditions is shown here for explanation.

In the presence of acid, the keto form undergoes tautomerization to become the enol form, which is planar and achiral. When enol converts back to keto, the protonation of the double bond could take place on either side of the plane, therefore producing equal amounts of both enantiomers for the keto form, that is the racemic mixture. So the solution becomes optical inactive.

