Chapter 5: More Reactions on Aromatic Compounds
5.4 Synthesis of Benzene Derivatives
5.4.1 Applications of EAS in Synthesis
EAS reaction on benzene is a powerful tool in the synthesis of benzene derivatives with a variety of structures. In planning the synthesis route, some considerations need to be accounted for.
- Looking at the activating/directing effects of substituents, to decide the proper order for carrying out the different EAS reactions, as in the example given below.
Examples
Propose a synthesis route for the following benzene derivative.
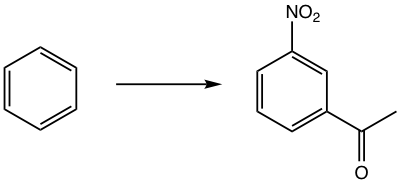
To synthesize the target compound, it seems that does not matter to introduce which group first since both NO2 and COCH3 are m-directors. A further look, however, indicates that NO2 is a strong deactivating group and makes the second EAS reaction much more difficult. So it is more reasonable to do Friedel-Drafts acylation first, then nitration.
Answer:
- Consider the reactivity and/or side reactions, for example, the limitations for Friedel-Crafts alkylation.
Refer to the example given in section 4.7.3.
- To synthesize the sterically hindered isomer, apply the blocking/deblocking strategy, as in the example given below.
Examples
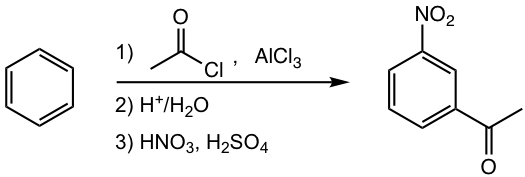
Propose a synthesis route for the following benzene derivative.
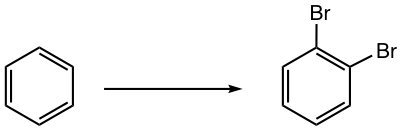
Bromine is an ortho-para director. When the 2nd bromination occurs, the 2nd Br atom goes to both ortho- and para-positions, with para-isomer slightly favored because of the steric hindrance on ortho-position. How to synthesize the o-dibromobenzene exclusively, without getting p-dibromobenzene? The strategy of introducing -SO3H as a blocking group is what can be applied for such a purpose.
Answer:
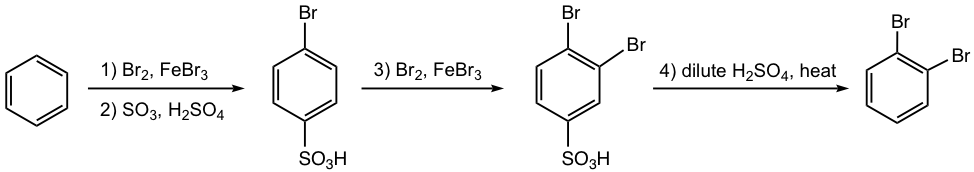 The sulfonation of bromobenzene occurs only on the para-position, because of the large size of the SO3H group. Since the SO3H group blocks the para-position of bromobenzene, the 2nd Br group will be introduced to ortho-position exclusively. The final product is obtained by the removal of the blocking group SO3H by heating in an acidic solution.
The sulfonation of bromobenzene occurs only on the para-position, because of the large size of the SO3H group. Since the SO3H group blocks the para-position of bromobenzene, the 2nd Br group will be introduced to ortho-position exclusively. The final product is obtained by the removal of the blocking group SO3H by heating in an acidic solution.
- Convert a strong activating group to a milder one, as in the example given below.
As mentioned in section 5.1.2, direct bromination of phenol results in 2,4,6-tribromophenol because of the strong activating property of the OH group. To avoid the undesired reactions, the strong activating groups such as OH or NH2 can be converted to a milder activating group, such as ester (OCOR) or amide (NHCOR) respectively. At the end of the reaction, these groups will be converted back to the original version.
Examples
Propose a synthesis route for the following benzene derivative.
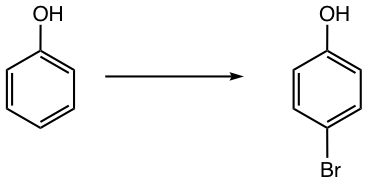
Answer:
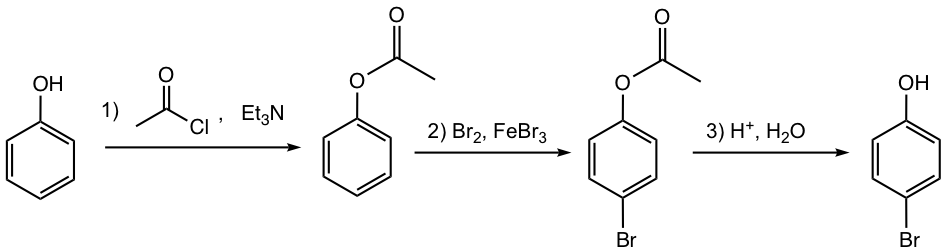
The reactions for preparing ester from alcohol, and the hydrolysis of ester to alcohol will be discussed in detail in Chapter 7.
5.4.2 Reaction of the Alkyl Substituents on Benzene Derivatives for Alkylbenzenes
Other than the EAS reactions, the conversions of substituents on the benzene ring to other functional groups also help to expand the scope for the synthesis of benzene derivatives. The reactions of the alkyl substituents of alkylbenzene will be summarized here. You will find that some of the following examples involve the reactions we have learned in earlier chapters, and a few brand-new reactions will be added.
Halogenation of the Alkyl substituent for Alkylbenzene (Radical)
Radical halogenation takes place on alkane, so it also occurs on the alkyl substituent on alkylbenzene. The benzylic position is the first choice for this reaction because of the special stability of the benzylic radical (refer to section 9.3 in Book I, and section 3.2).
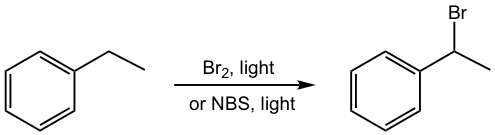
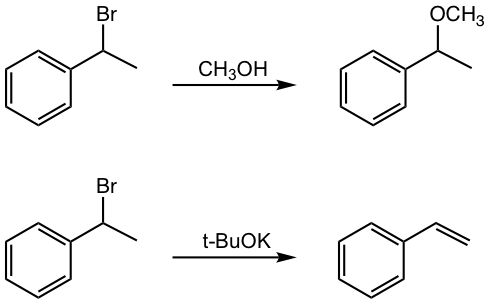
Once the halogen is introduced into the alkyl substituent, the benzylic halide could undergo substitution or elimination as other halides, examples are given in Fig. 5.4b.
Oxidation of the Alkyl substituent for Alkylbenzene
The alkyl substituent on alkylbenzene can be oxidized by a strong oxidizing agent to a carboxylic acid (COOH) group. Two strong oxidizing agents can be used for this reaction: KMnO4 in basic condition, or Na2Cr2O7 in acidic solutions.
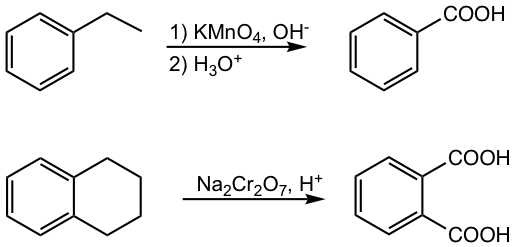
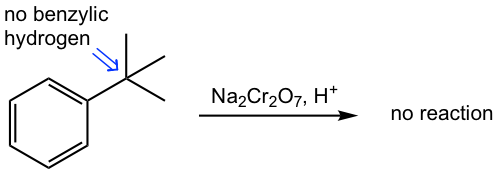
The initial step of the reaction is that the oxidizing agent abstracts a benzylic hydrogen, therefore only the alkyl group with the hydrogen on the benzylic position undergoes the oxidation.
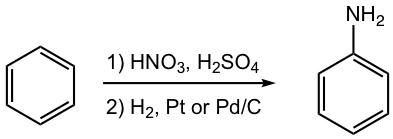
Conversion of NO2 to NH2 group
As we will learn in the next section 5.5, aniline is an important benzene derivative that has special application in synthesis. However, the NH2 group cannot be introduced to the benzene ring directly. Instead, aniline can be prepared by reduction of nitrobenzene.