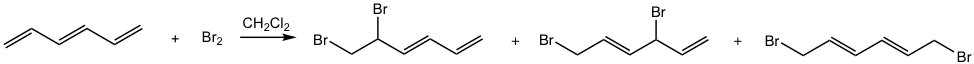Chapter 3: Conjugated Unsaturated Systems
3.4 Reactions of 1,3-Butadiene
Electrophilic addition is still the most common and important reaction to conjugated diene, as for other regular alkenes (refer to section 10.2 in Book I), however, with a mixture of products produced. The reaction of 1,3-butadiene with HBr is shown in Fig. 3.4a.
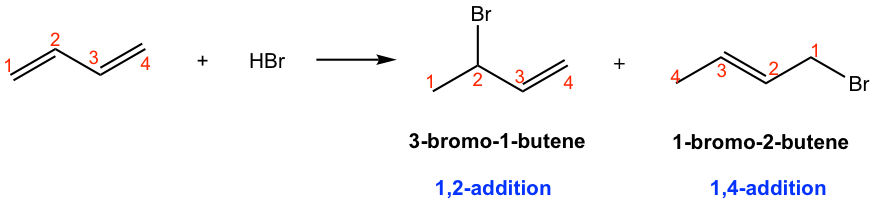
In the first product, 3-bromo-1-butene, small molecule HBr is added to C1 and C2, so the product is called 1,2-addition product. For the addition that produces 1-bromo-2-butene, HBr is added to C1 and C4 positions, and this is called a 1,4-addition product.
The 1,2-addition product is what we would expect, which comes from the addition of HBr to the C=C bond by following Markovnikov’s rule. But how about the second product 1-bromo-2-butene? Other than addition, why does the position of the C=C bond move as well? Proposing the reaction mechanism helps to solve the questions.
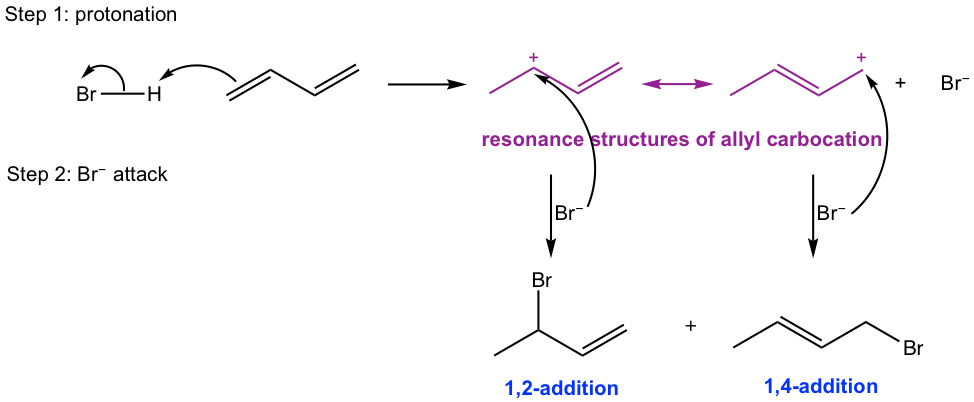
The overall mechanism involves two parts: protonation and nucleophilic addition. In the first protonation step, the double bond is protonated by strong acid HBr, and an allyl carbocation intermediate is formed. Because of the special property for allylic carbonation, as we learned in section 3.1, another resonance structure is also available that helps to stabilize the allyl cation. In the second step, the addition of bromide Br- could occur on either of the two allyl carbocations and lead to the formation of two addition products.
As a general trend for electrophilic addition of conjugated diene, a mixture of 1,2- and 1,4-addition products is formed. Further studies indicate that the ratio of 1,2-addition versus 1,4-addition product depends on the reaction temperature. At low temperatures, the 1,2-addition product (kinetic control product) is the major one, while the 1,4-addition product (thermodynamic control product) dominates at high temperatures.

Kinetic Control and Thermodynamic Control of Electrophilic Addition to Conjugated Diene
The phenomenon that the amount of 1,2-addition and 1,4-addition product is temperature-dependent, is a great example of an important aspect of reactivity: kinetic control and thermodynamic control.
- Kinetic control is the pathway reaction proceeds at low temperature, that is also the path with smaller energy of activation barrier, therefore faster reaction rate (speed).
- Thermodynamic control means the reaction proceeds through a pathway that leads to the products with better stabilities (lower energy), and such a pathway has higher activation energy and is favored at high temperatures.
The difference between the two processes is illustrated in the reaction potential energy diagram in Fig. 3.4d. For the addition of 1,3-butadiene, the 1,2-addition product is the kinetic control product (blue pathway) and the 1,4-addition product is the thermodynamic control product (red pathway).
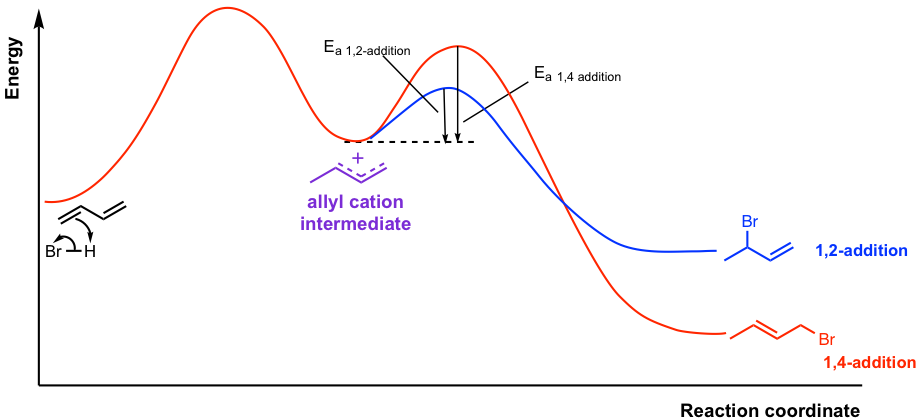
At low temperatures, there is not enough energy in the reaction system to have enough collisions capable of overcoming the higher energy barrier that leads to the formation of the 1,4-addition product, so the 1,2-addition product predominates because it needs less energy. The formation of 1,2-addition product is faster (low activation energy) and irreversibly at low temperatures.
At high temperatures, there is enough energy to have sufficient collisions that allow the formation of both 1,2- and 1,4-addition products. For such a case, the better stability of the 1,4-addition product (internal alkene with two substituents, lower energy) makes it predominates the equilibrium, and become the major product. Studies also show that when the product mixture from the low temperature (1,2-addition product as major) is warmed up to the higher temperature, the product distribution changes over time with the 1,4-product becoming predominant.
Examples
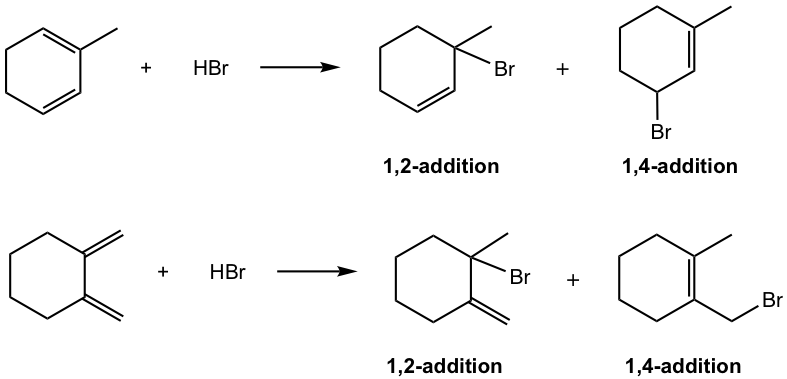
Show both 1,2- and 1,4- addition products of the following reaction.
Answers:
Reactions of Polyenes
The electrophilic addition of polyene proceeds in a similar way as for 1,3-butadiene, with the mixture of addition products obtained because of the non-equivalent resonance structures of allyl carbocation intermediates.