Chapter 2: Aldehydes and Ketones
2.1 Structures, Properties and Naming of Aldehydes and Ketones
For alcohol preparations, we have had some discussions about the reactions of aldehydes and ketones in section 1.4 of Chapter 1. Here we will get more insights into the the chemistry properties of aldehydes and ketones, which are the foundations of other reactions will be covered in this and the following chapters.
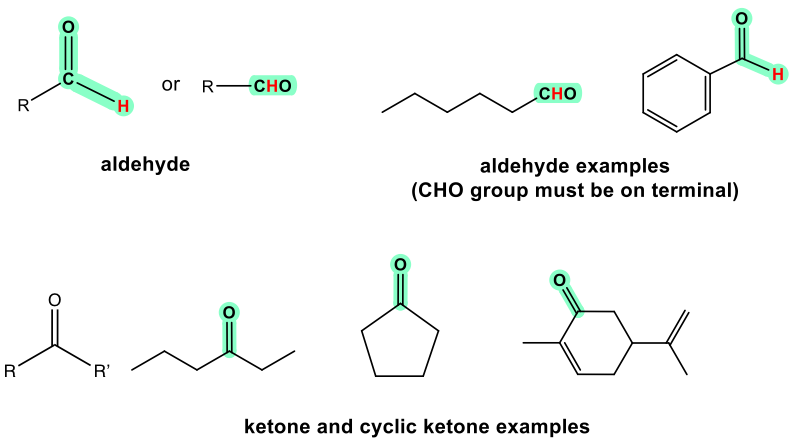
Both aldehydes and ketones contain the carbonyl (C=O) group, while the aldehyde must have at least one hydrogen atom bond with the carbonyl carbon, and ketone must have two alkyl (aryl) groups that could be either same or different, as shown in Fig. 2.1a.
Because of the polarity of the C=O bond, the carbonyl group is polar, and so are the aldehyde and ketone molecules. Dipole-dipole force is the major intermolecular force applied to aldehydes and ketones, as such the boiling points of aldehydes or ketones are higher than that of the hydrocarbon with a similar molar mass. The polarity however decreases with the carbon chain increase, since the polarity of the C=O bond is overridden by the non-polar hydrocarbon chain.
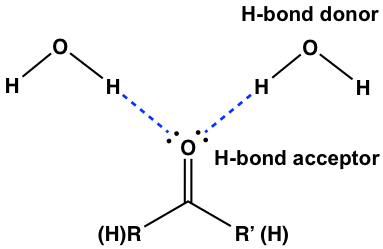
Even though hydrogen bonding does not apply between aldehyde and ketone molecules, they are good hydrogen bond acceptors. The two electron pairs on the oxygen atom of the carbonyl group are ready to form H-bonds with water molecules. That explains the good solubility of smaller size aldehydes and ketones.
The physical properties of some common aldehydes and ketones are listed in Table 2.1.
|
Name |
Structure |
Boiling Point (°C) |
Solubility in water |
| Formaldehyde | 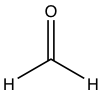 |
-21 | infinite |
| Acetaldehyde | 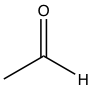 |
21 | infinite |
| Propanal | 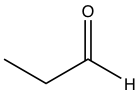 |
49 | very soluble |
| Acetone | 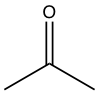 |
56 | infinite |
| Butanone | 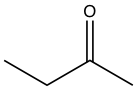 |
80 | very soluble |
| Benzaldehyde |  |
178 | slightly soluble |
Table 2.1 Physical properties of some common aldehydes and ketones
Simple aldehydes and ketones can be named by following the IUPAC rules we learned before (section 2.4 of Book I), with a couple of highlights here:
- Change the ending of the parent alkane/alkene/alkyne to the al for aldehyde, and one for ketone;
- The suffix carbaldehyde is added when the -CHO group is attached to a ring.
Several examples are given here to illustrate the IUPAC naming of aldehydes and ketones.
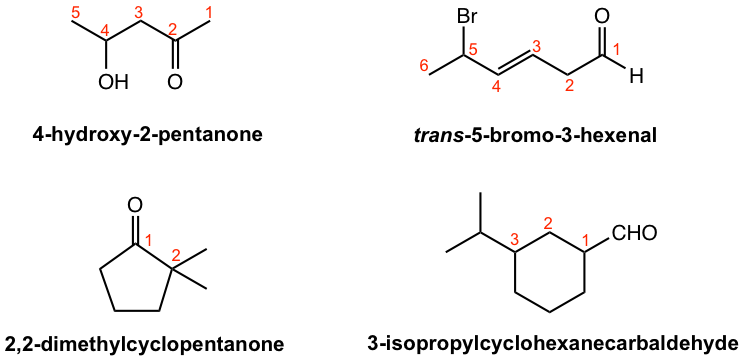
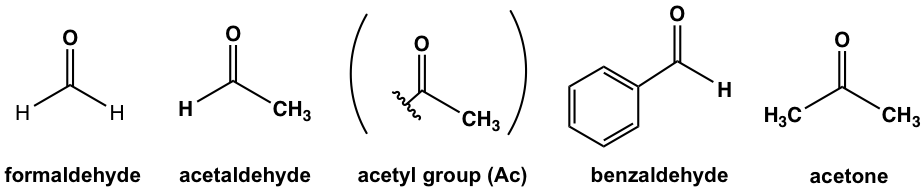
The common names have been adopted broadly (in this book as well) for following aldehydes and ketones.