Chapter 1: Alcohols and Ethers
1.5 Williamson Ether Synthesis
Here we will only have discussions on Williamson ether synthesis, the most well-known and effective method for the synthesis of unsymmetrical ethers. The reaction goes through the SN2 mechanism (refer to section 7.3 in Book I), with alkoxides (act as nucleophiles) reacting with substrates that bear a good leaving group.
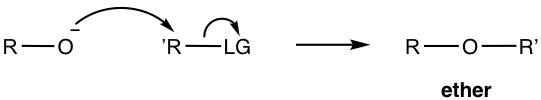
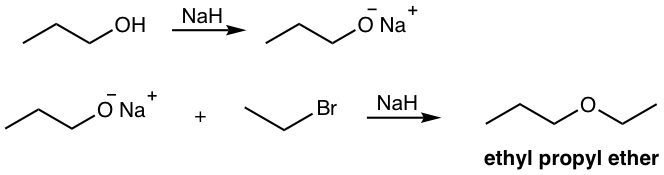
Because of the SN2 nature of the reaction, primary or secondary substrates are typical for Williamson synthesis, with primary (and methyl) substrates giving the best results. Elimination occurs for tertiary substrates. Alkoxide can be produced by treating the alcohol with sodium or sodium hydride. An example of the synthesis of ethyl propyl ether from propanol and ethyl bromide is shown below.
Examples
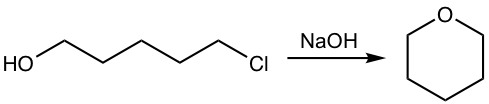
Show the product of the following reaction.
![]()
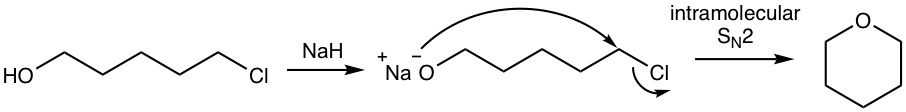
Analysis: Alkoxide is produced from the reaction of alcohol (OH group) with NaH, the alkoxide then reacts with chloride by intramolecular SN2 mechanism to give the cyclic ether.
Answer:
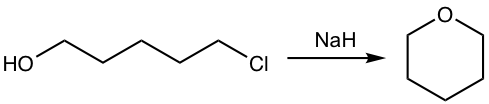 NaOH works for the above synthesis as well. Even the reaction between alcohol and NaOH does not favor the large equilibrium concentration of alkoxide, the fast intramolecular reaction of alkoxide with chloride helps to drive the equilibrium to the product side.
NaOH works for the above synthesis as well. Even the reaction between alcohol and NaOH does not favor the large equilibrium concentration of alkoxide, the fast intramolecular reaction of alkoxide with chloride helps to drive the equilibrium to the product side.