Chapter 3: Conjugated Unsaturated Systems
To introduce the conjugated system, let’s first take a look at a reaction.
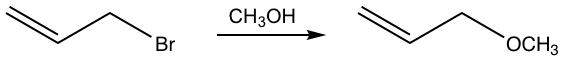
This is a substitution reaction, and it proceeds well with good yield. The question is whether the reaction goes through SN1 or SN2 mechanism. The weak nucleophile CH3OH seems like a suitable choice for SN1, but the primary bromide substrate has a high tendency to go with SN2. What is the correct answer then?
It turns out that the reaction goes with the SN1 mechanism, with the stable allyl carbocation involved as the intermediate. The allylic position is the carbon which is adjacent to a C=C double (π) bond. Although it is a primary carbocation, the allyl carbocation shows special stability. Studies indicate that allyl carbocation is even more stable than a 2° carbocation and is almost as stable as a 3° carbocation due to the resonance stabilization effect of the double bond.
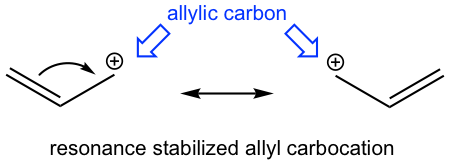
Another example of the special reactivity on allylic position is the radical halogenation of propene, in which allyl radical is the intermediate involved. Allylic hydrogens are especially reactive in radical substitution reactions, despite the fact that they are bonded on a primary carbon.

The reason for the special stability of allyl carbocation and allyl radical is that they both belong to a special system called the conjugated system. The special properties and reactivities of the conjugated system are the topics for this chapter.

