Chapter 9: Carbohydrates
9.3 Other Reactions of Monosaccharides
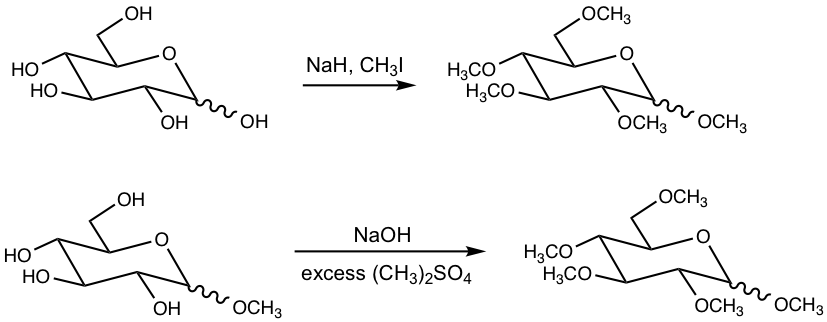
9.3.1 Alkylation (Formation of Ethers)
The hydroxyl groups of monosaccharides can be converted to ethers by a couple of different conditions, and methyl ethers are prepared most commonly. The reagents that can be used to synthesize methyl ethers include:
- NaH and CH3I
- NaOH and (CH3)2SO4
- Ag2O and CH3I
Although different conditions could be applied, the reactions essentially go through SN2 where the OH groups are deprotonated first and then react with methylation reagents. A couple of examples are given below.
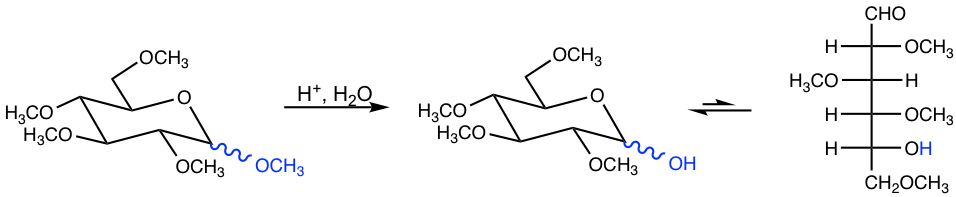
A specific note is that all the OH groups are methylated with the above conditions to produce pentamethyl derivative, while the reaction with CH3OH/H+ (in section 9.2.3) only converts the OH on anomeric carbon to OCH3 (hemiacetal to acetal). On the other side, when the pentamethyl derivative of monosaccharide is treated in an acidic solution, only the methyl group on anomeric carbon is hydrolyzed because it is an acetal, and all the other ethers are not affected by aqueous hydrolysis.
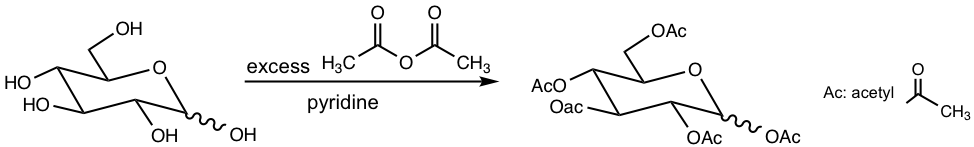
9.3.2 Acylation (Formation of Ester)
The hydroxyl groups of monosaccharides can be converted to esters by reacting with excess anhydride, in the presence of a weak base (refer to section 7.5.2, the preparation of ester from anhydride).
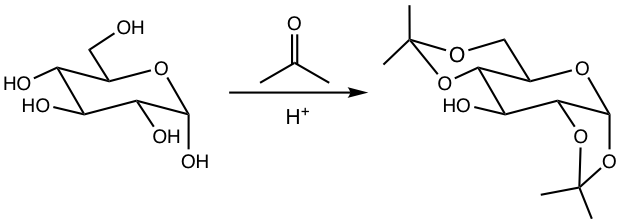
9.3.3 Conversion to Cyclic Acetals
There are multiple hydroxyl groups in monosaccharides, and they are good candidates as 1,2-diol (or 1,3-diol) to produce cyclic acetals with aldehyde or ketone, as the example given in Fig. 9.3d. Such a reaction only occurs when the two OH groups are cis to each other in terms of the ring.