Chapter 9: Carbohydrates
9.1 Classification and Configurations of Carbohydrates and Monosaccharides
Carbohydrates are polyhydroxy aldehydes and polyhydroxy ketones, and compounds formed by linking polyhydroxy aldehydes or polyhydroxy ketones together. The term carbohydrate and saccharide could be used interchangeably. Saccharide comes from the word for sugar in several early languages, for example, sakcharon in Greek and saccharum in Latin.
The simplest carbohydrates are called monosaccharides. The complex carbohydrates contain two or more monosaccharides linked together. Disaccharides have two monosaccharides, trisaccharides have three monosaccharides, and so on. Oligosaccharides have 2-10 molecules of monosaccharides, and polysaccharides have 10 or more monosaccharides. Complex carbohydrates can be broken down into monosaccharides by hydrolysis.
9.1.1 Classification of Monosaccharides
Monosaccharides can be classified in different ways, which is summarized in Table 9.1.
- By the number of carbon atoms present in the molecule.
- Whether the molecule contains an aldehyde or a ketone functional group. The monosaccharide containing an aldehyde group is called an aldose, and one containing a ketone group is called a ketose.
- Pyranose and furanose for cyclic structure of monosaccharide, refer to section 9.2 for details.
| 3-carbon | 4-carbon | 5-carbon | 6-carbon | 7-carbon | |
| Triose | Tetrose | Pentose | Hexose | Heptose | |
| Aldehyde: | Aldotriose | Aldotetrose | Aldopentose | Aldohexose | Aldoheptose |
| Ketone: | ketotriose | ketotetrose | ketopentose | ketohexose | ketoheptose |
Table 9.1 Classification of monosaccharides
9.1.2 The D and L Notation and Configurations of Monosaccharides
The simplest monosaccharides have three carbons, they are glyceraldehyde (aldotriose) and dihydroxyacetone (ketotriose). They are also the only two saccharides whose names do not end in “ose”. Glyceraldehyde has one chirality center, and dihydroxyacetone has no chirality center.
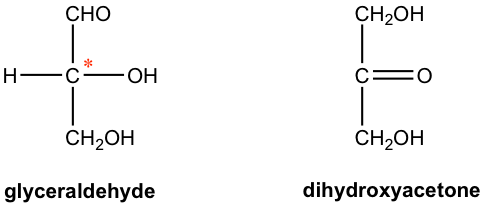
Since there is one chirality center in the glyceraldehyde, it has two enantiomers. The structures of both enantiomers are shown in Fig. 9.1b in Fisher projection, with configuration and optical rotation designation for each given as well. The R/S designation is determined according to the Cahn-Ingold-Prelog rule (refer to section??? Book I), and (+) and (-) for optical activity come from experimental results. Fischer projection is the most common way employed in carbohydrate chemistry to show the chemical structures of open-chain saccharides, in which chirality center(s) are usually involved.
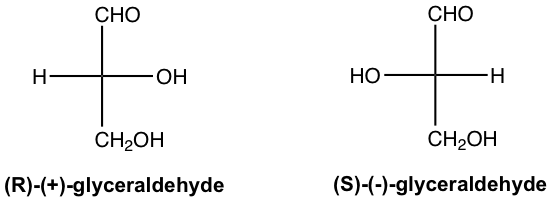
Early in the twentieth century, before the absolute configuration of any organic compounds could be determined, another system of stereochemical designation was introduced for monosaccharides. In this system, the carbonyl group is always placed on top (for aldose) or as close to the top as possible (for ketose) in a Fisher projection. If the OH group attached to the bottom-most chirality center (the carbon second from the bottom) is on the right side of the Fisher projection, the compound is a D-saccharide. If that OH group is on the left of the Fisher projection, the compound is a L-saccharide. Based on the D/L designation, the R-glyceraldehyde is the D-glyceraldehyde, and the S-glyceraldehyde is the L-glyceraldehyde. The D/L notation of stereochemical designations is thoroughly employed in carbohydrate chemistry, we will follow this convention in this book as well.
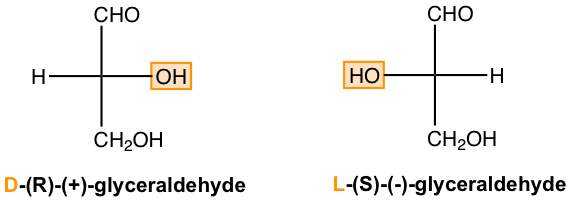
There are several points to be noted for the D and L notations:
- D-monosaccharide and L-monosaccharide are mirror images, or enantiomers, to each other.
- Almost all sugars found in nature are D-saccharides.
- Like R/S, the D/L notations only indicate the configuration of the chirality center for the compound, they do not indicate the optical activity of the compound. As such, there is no correlation between D/L and (+)/(-).
Aldoses
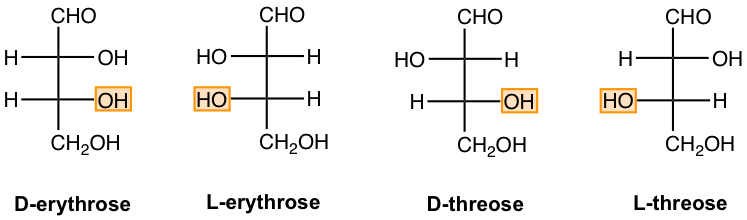
For aldotetrose, there are two chirality centers and therefore four stereoisomers. As shown in Fig. 9.1d, two of them are D-sugars, and two are L-sugars. Each aldotetrose has its name.
With the number of carbons increasing to 5 (aldopentose) or 6 carbons (aldohexose), the number of diastereomers increases to eight and sixteen respectively. The monosaccharides (up to six carbons) belonging to the D-family are shown in the Fig. 9.1e below. The D-(+)-glucose is the most common example.
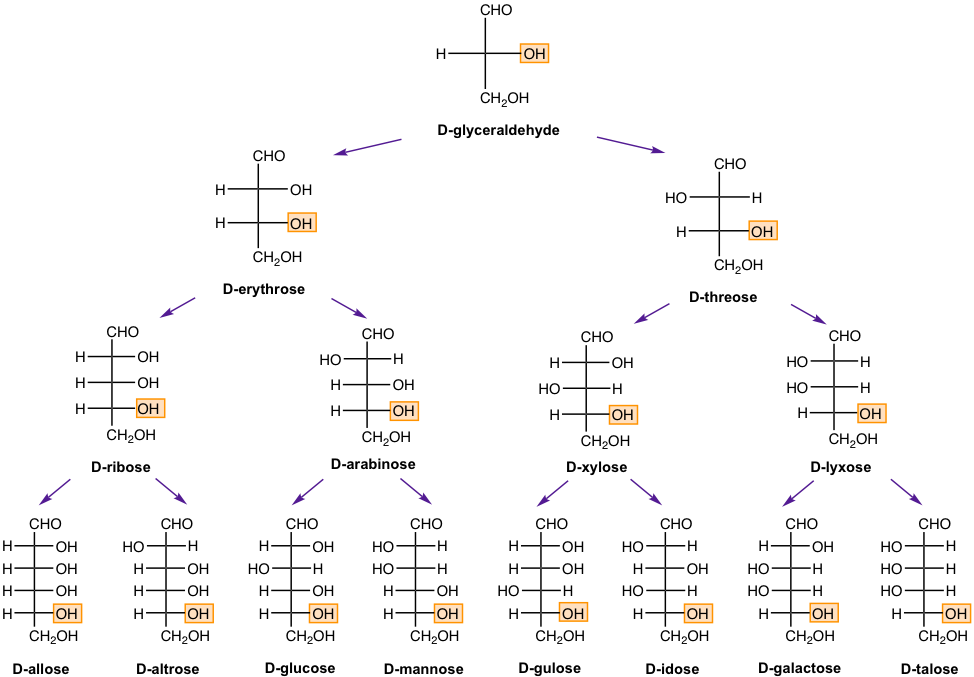
The diastereomers that differ in configuration at only one chirality center are called epimers. For example, D-glucose and D-mannose are C-2 epimers, and D-glucose and D-allose are C-3 epimers.
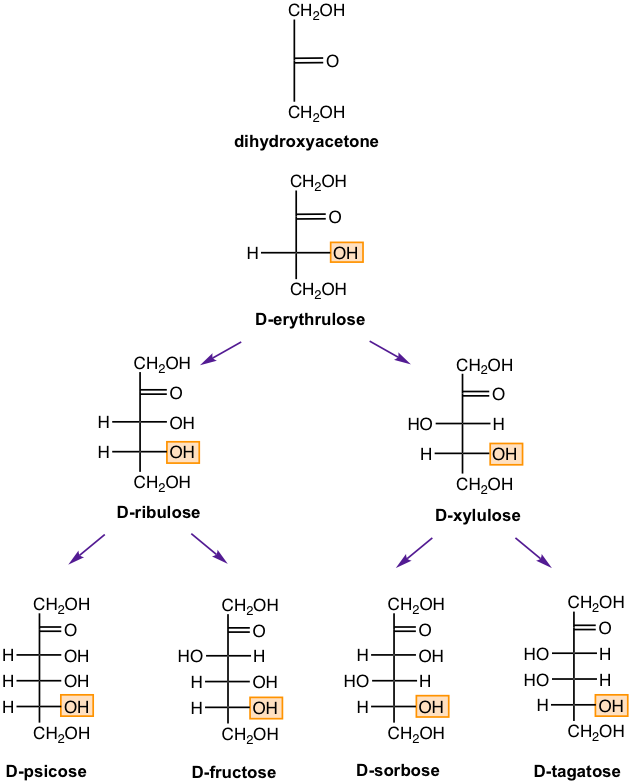
Ketoses
Carbohydrates that have a ketone group at the C-2 position are called ketoses. A ketose has one less chirality center than an aldose with the same number of carbons. The structures of D-ketoses up to six carbons are shown in Fig. 9.1f. D-fructose is the sugar that exists in fruits.