Chapter 8: The Claisen Condensation and Applications in Synthesis
8.1 Claisen Condensation Fundamentals
The general reaction equation of Claisen condensation is shown in Fig. 8.1a. Please note that the overall reaction condition involves an acidic work-up process, the reason for this will be explained in the mechanism. In a Claisen Condensation, the enolate of one ester molecule (black one) acts as the nucleophile to do acyl substitution (nucleophilic addition-elimination) to another ester molecule (blue one). A new C-C bond (red bond) connects the two units together and β-keto ester is the product produced. In Chapter 6 we saw how β-keto esters are useful in synthesis, therefore Claisen condensation is a reaction that has important and broad applications in synthesis.
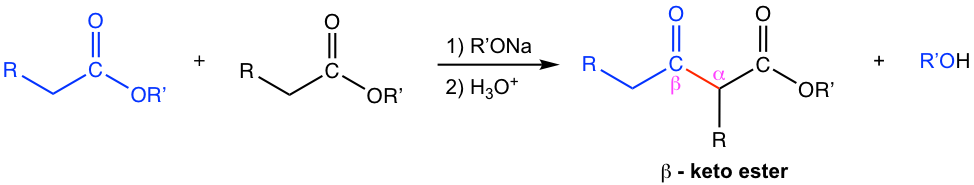
The detailed reaction mechanism is shown in Fig. 8.1b.
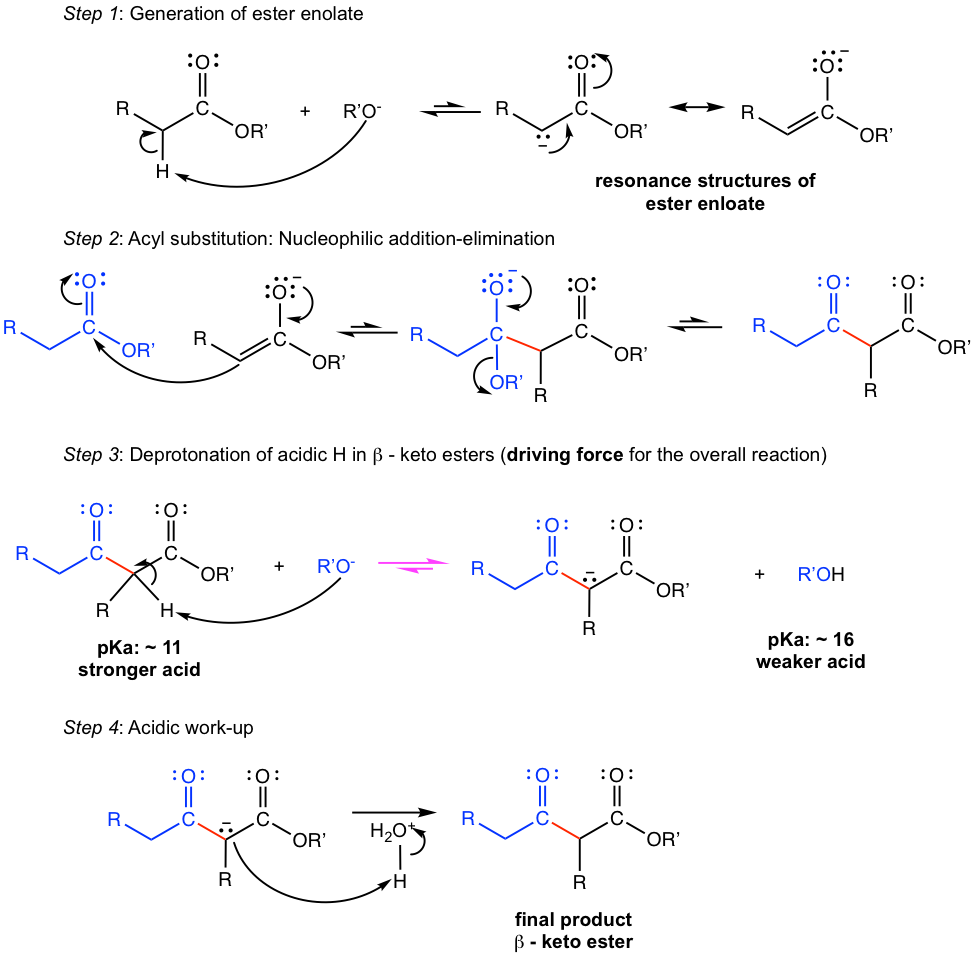
Further explanations of the mechanism:
- The enolate of one ester molecule is generated in the first step, and then acts as the nucleophile to attack the carbonyl group of another ester molecule. The 2nd ester molecule undergoes the acyl substitution by getting rid of the R’O–, and produces the β-keto ester in step 2.
- Special attention: both equilibria in steps 1 and 2 are unfavorable towards the product. In step 1: the α-hydrogen in ester is a weaker acid than an alcohol. In step 2, the R’O– group is a poor leaving group. To draw the overall equilibrium towards the product side, another deprotonation step (step 3) of the β-keto esters is necessary.
- The α-hydrogen in β-keto esters is more acidic (with pKa of about 11) than alcohol (pKa is about 16), therefore R’O– is a base that is strong enough to deprotonate the α-hydrogen in β-keto esters. As a result, step 3 is highly favorable and draws the overall equilibrium towards the product side. The Claisen condensation reaction will not be productive without this critical step. The neutral final product is formed by the acidic work-up step in step 4.
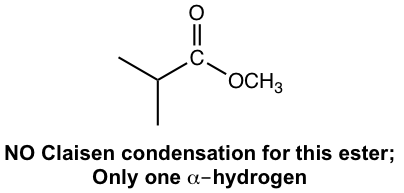
- Ester that has only one α-hydrogen therefore does not undergo Claisen condensation, because it does not have acidic α-hydrogen to be deprotonated in step 3 of the mechanism.
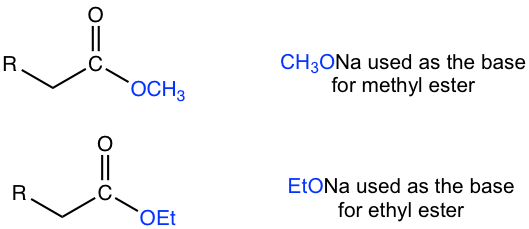
- Alkoxide is usually used as the base in Claisen condensation. The alkyl group in alkoxide should be the same as the alkoxyl group of the ester, both shown as R’O in the above general equation and mechanism. Hydroxide (OH–) cannot be used as a base for Claisen condensation because it could react with the ester for hydrolysis.