Chapter 7: Carboxylic Acid and Derivatives
7.8 Reactions of Nitriles
7.8.1 Hydrolysis
As mentioned at the beginning of this chapter, nitriles are categorized as carboxylic acid derivatives because they can be converted to carboxylic acid by hydrolysis.

The mechanisms for the hydrolysis of nitrile, in either acidic or basic conditions, are related to the hydrolysis of amide. We will focus on the acidic hydrolysis mechanism (Fig. 7.8b) in this book.
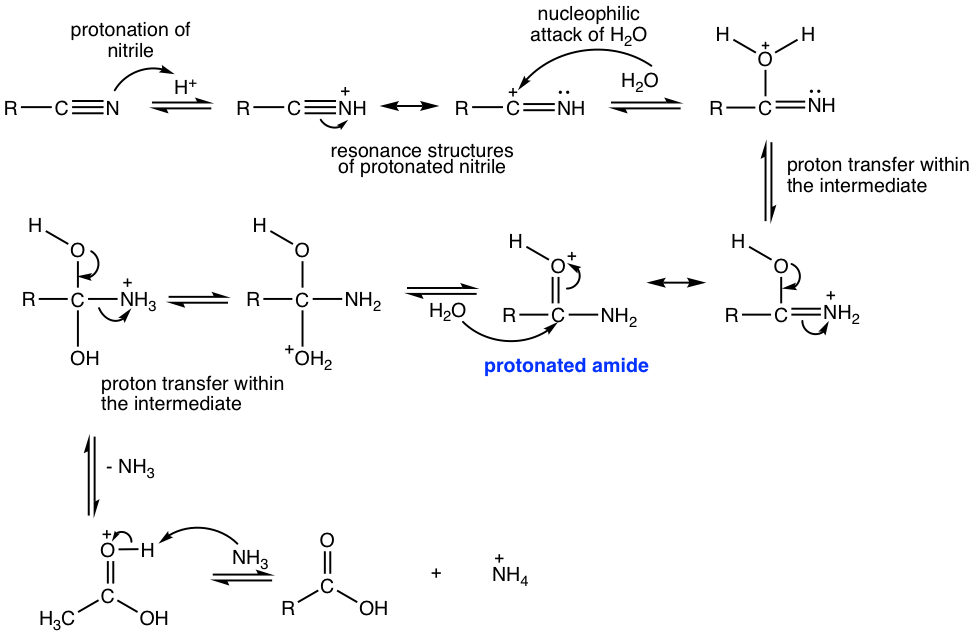
The mechanism for acidic hydrolysis of nitrile involves a couple of key steps as summarized below. Please note that from the step in which protonated amide is formed, the following mechanism is just the acidic hydrolysis of amide.
- Protonation of the nitrile group. The protonation increases the polarity of the carbonyl group and makes the carbon atom more susceptible to nucleophilic attack by water, which is a weak nucleophile.
- Nucleophilic attack of water to protonated nitrile.
- Proton is first lost from the oxygen atom and then gained by the nitrogen atom, and the resonance structure after the proton transfer gives a protonated amide.
- Acidic hydrolysis of amide to produce carboxylic acid.
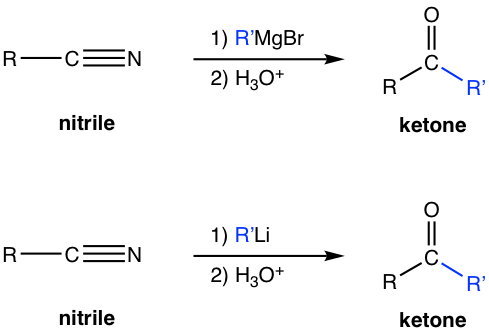
7.8.2 Reaction of Nitriles with Organometallic Reagents
The reaction of nitrile with Grignard reagent or organolithium reagent produces ketone as the final product.
7.8.3 Reduction
Reduction of nitrile with LiAlH4 gives a primary amine, RCH2NH2. The reaction occurs by the addition of H– to CN triple bond twice to form a dianion, which is then protonated by water to give the amine.
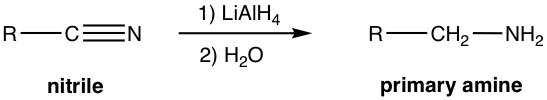
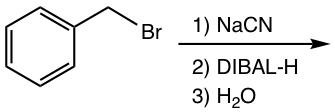
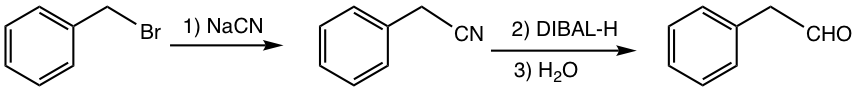
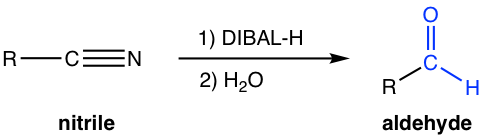
Reduction of nitrile with milder reducing agent DIBAL-H produces aldehyde.
Examples