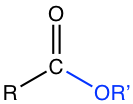
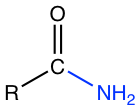
Chapter 7: Carboxylic Acid and Derivatives
7.7 Other Reactions of Carboxylic Acid Derivatives
7.7.1 Hydrolysis of Carboxylic Acid Derivatives
The base-promoted hydrolysis of ester represents a specification process of saponification and was discussed in section 7.5.2. Actually, all the carboxylic acid derivatives could undergo a hydrolysis reaction, that is reacting with water to produce carboxylic acid and the other components.
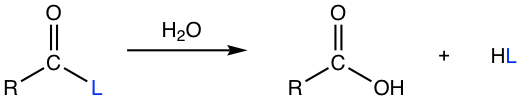
The mechanism of the hydrolysis also follows the nucleophilic addition-elimination process generally, therefore the tendency for derivatives to be hydrolyzed also decreases with the order from acyl halide, anhydride, ester to amide. As a result, the reaction condition required for hydrolysis varies for different derivatives, and they are summarized in Table 7.2 below.
| Overall reaction |  |
|||
|---|---|---|---|---|
| Derivatives |
Acyl halide |
Anhydride |
Ester |
Amide |
| Reaction Conditions | Fast reaction at room temperature, no catalyst required | Room temperature, no catalyst required | Need catalyst (H+ or OH–) and heat | Need catalyst (H+ or OH–) and heat |
Table 7.2 Reaction conditions for hydrolysis of carboxylic acid derivatives
Acyl chlorides and anhydrides could be hydrolyzed so easily, that no catalyst was required. For esters and amides, the hydrolysis requires either acid or base as the catalyst, together with heating. Hydrolysis reaction sometimes can be used to prepare certain products, for example, saponification, or hydrolysis of nitrile to produce carboxylic acid. For most cases, however, hydrolysis is something that chemists would need to avoid because it destroys the valuable derivative and converts it back to carboxylic acid. For this reason, some reactive derivatives such as acyl chloride and anhydride are usually stored under specially controlled dry conditions to keep moisture away.
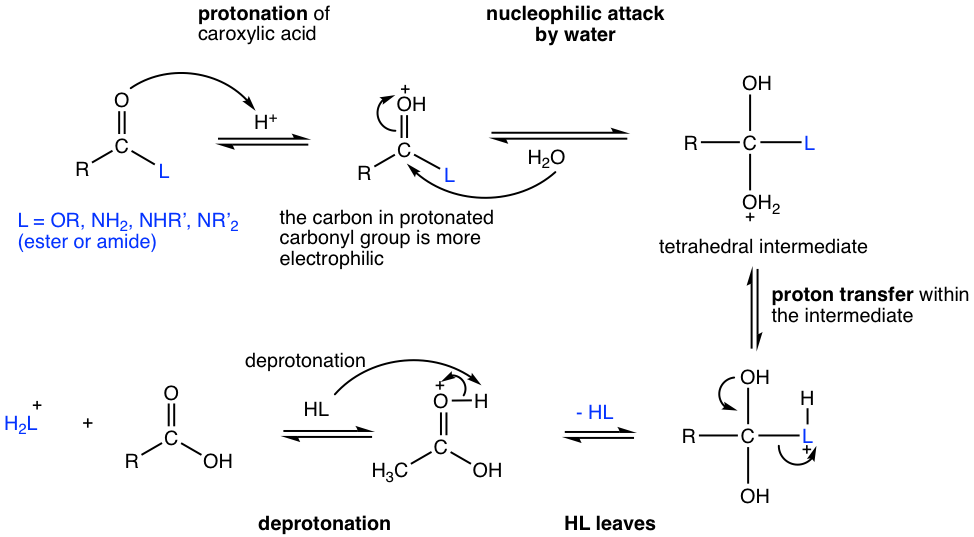
The mechanism for acidic-catalyzed hydrolysis of ester and amide is shown in Fig. 7.7b. You may notice that the mechanism is very similar to the esterification mechanism in Fig. 7.5b. The two mechanisms are reverse processes to each other, and both involve the same steps of protonation – nucleophilic attack – proton transfer – leaving group leaves – deprotonation. In hydrolysis, water acts as a nucleophile to attack the protonated carbonyl group, and then HL (ROH for ester or RNH2 for amide) leaves as a leaving group in the later step.
The hydrolysis of ester under basic conditions is also called saponification, as we learned in section 7.5.2. The mechanism (shown in Fig. 7.5e) follows the general acyl substitution process involving nucleophilic attack and leaving group leave. The hydrolysis of amide under basic conditions generally follows the same mechanism.
Examples
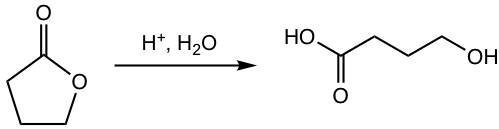
Show the hydrolysis product:
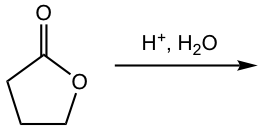
Answer:
7.7.2 Reaction of Carboxylic Acid Derivatives with Organometallic Reagents
Grignard Reagent
When we learned about the Grignard reaction (section 1.5.4), we mentioned that esters react with Grignard reagents. Other derivatives including acyl chloride and anhydride react with Grignard reagent as well. All these derivatives react with two equivalents of Grignard reagent and produce alcohols. The general reaction equation and mechanism are shown here.
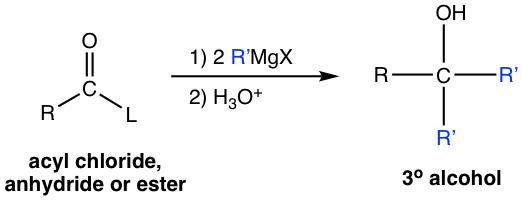
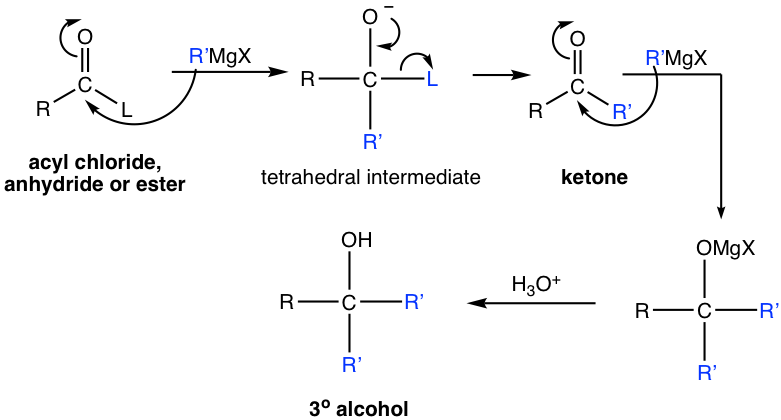
As it is illustrated in the above mechanism the derivative first reacts with one equivalent of R’MgX , by acyl substitution, to generate ketone. Then ketone reacts with another equivalent of Grignard reagent R’MgX, by nucleophilic addition, and gives the final product — tertiary (3°) alcohol.
Organocuprate Reagent
Organocuprate reagent (refer to section 6.5.3 for preparation of organocuprate reagent) also reacts with acyl chloride for acyl substitution. Because of the low reactivity of organocuprate, however, it does not react with ketone anymore, so ketone is obtained as the final product.

Reducing Agent: LiAlH4 and DIBAL-H
Lithium aluminum hydride, LiAlH4, is a strong reducing agent (refer to section 1.4.2 for structure and property of LiAlH4), and reduces carboxylic acid, acyl chloride, and ester to alcohols (Fig. 7.78f). Please note that two alcohols are obtained for the reduction of ester with LiAlH4. Amides are reduced by LiAlH4 to amines.
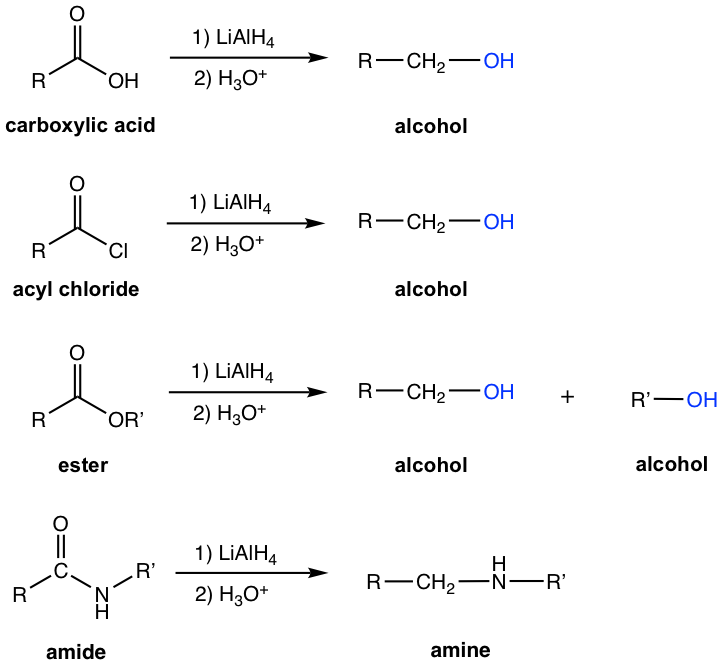
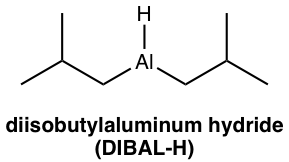
Diisobutylaluminum hydride (DIBAL-H, (i-Bu)2AlH) is a milder reducing agent than LiAlH4. In the structure of DIBAL-H, two hydrogens in aluminum hydride are replaced by bulky isobutyl groups, and the steric hindrance results in lower reactivity. The reduction of carboxylic acid derivatives with DIBAL-H produces aldehyde as the final product.