Chapter 6: Reaction at the α-Carbon of Carbonyl Compounds
6.4 Aldol Reactions
6.4.1 Aldo additions and aldol condensations
Another very important type of reaction to build a carbon-carbon bond by applying enolate is the aldol reaction, which includes aldol additions and aldol condensations.
Aldol reaction is the reaction between enolate (or enol), which acts as a nucleophile, and the electrophilic carbonyl group of the other aldehyde or ketone molecule. Since enolate also comes from the deprotonation of aldehyde or ketone, the aldol reaction is the reaction between two molecules of carbonyl compounds, as shown below in Fig. 6.4a.
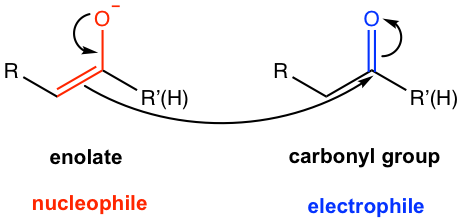
Aldol addition is the reaction in which the two carbonyl compounds are added together, and produce β-hydroxy aldehyde or ketone as a product. The name “aldol” comes from b-hydroxy aldehyde because it contains both an aldehyde and an alcohol functional group.
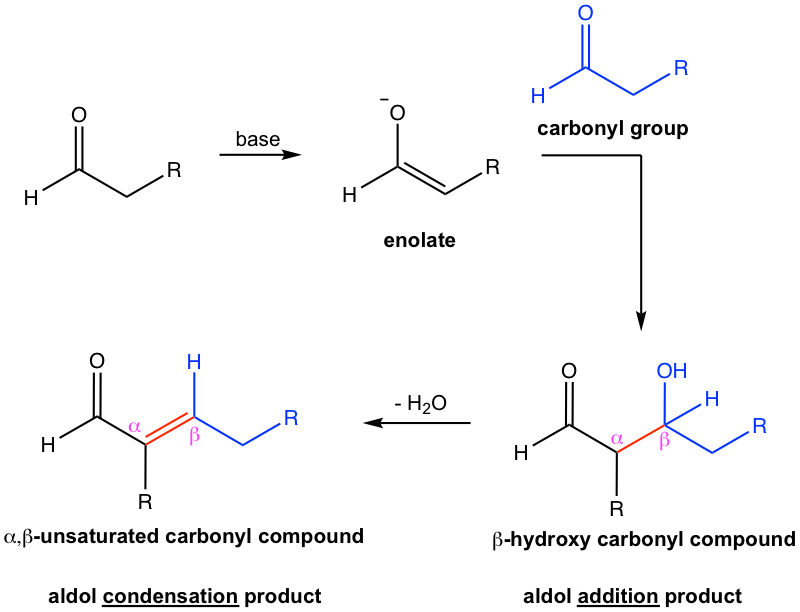
The initial aldol addition product could also undergo a dehydration reaction, and give α,β-unsaturated aldehyde/ketone, and then the overall reaction is an aldol condensation.
We will take a closer look at the reaction mechanism for aldol reactions.
Aldol Additions
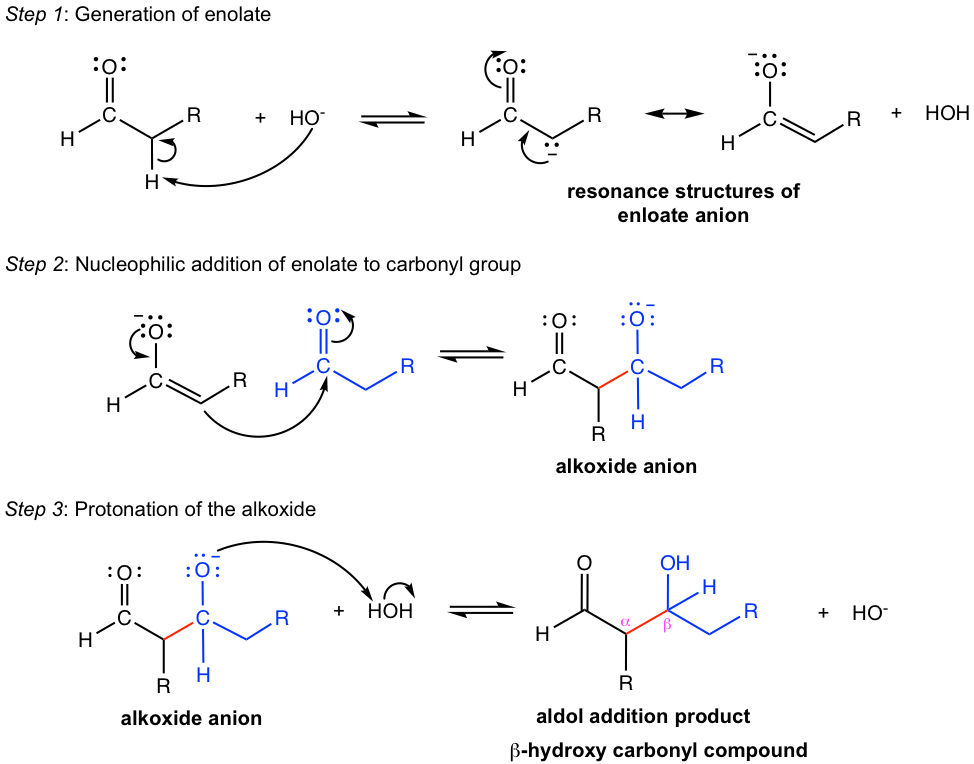
With hydroxide (OH–) or alkoxide (OR–) commonly used as bases, aldol addition is a reversible reaction that stays at equilibrium. The decent yield of aldol addition product could be obtained for aldehydes, because of the higher reactivity of carbonyl group in aldehyde. Ketones are less susceptible to aldol addition due to lower reactivity. However, as we will see in the next section dehydration of the aldol addition product helps the equilibrium to move towards completion, for both aldehydes and ketones.
Examples
Show the aldol addition product of the following reaction.
Tips on how to figure out the aldol addition product:
An important/useful hint is that the addition product is like an “adduct”, with a new C-C bond formed between the α-carbon of one molecule and the carbonyl carbon of the other molecule, and the carbonyl group turns into an OH group. The total number of carbons in the product is the sum of the number of carbons in each molecule.
Strategy:
- Apply the hint mentioned above to connect the two molecules, as shown by the red dotted line below.
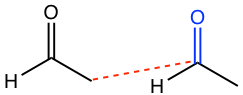
- Change the blue carbonyl to the OH group, and redraw the structure of the product in a reasonable way.
- Double-check the answer: Does the total number of carbons match? Is it a β-hydroxy aldehyde?
- – Yes, the product is a β-hydroxy aldehyde with a total of 4 carbons.
Answer:
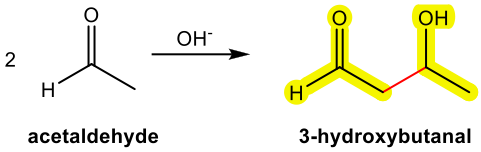
Aldol Condensations
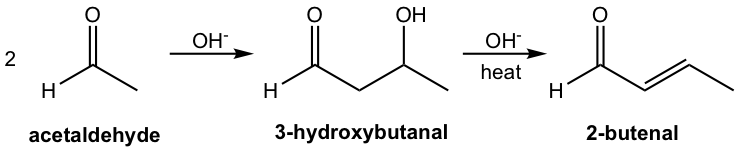
When the aldol addition product undergoes dehydration, an α,β-unsaturated aldehyde or ketone is formed and the overall reaction is called an aldol condensation. Because α,β-unsaturated aldehyde or ketone is a conjugated system with the C=C double bond conjugated with the carbonyl group, it shows special stability, and therefore dehydration occurs easily upon heating.
For example of the aldol addition product 3-hydroxybutanol in the previous example, dehydration under the basic condition leads to the formation of aldol condensation product 2-butenal (Fig. 6.4d). With low temperature, aldol addition product is obtained; and aldol condensation product is obtained with heating.
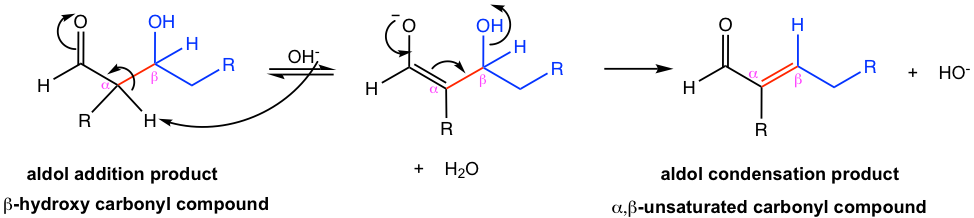
In the mechanism for dehydration (Fig. 6.4e), the α-hydrogen is deprotonated first, then the OH group leaves. The dehydration step is irreversible. The special stability of the conjugated system with alkene and carbonyl group is the driving force that leads to the completion of the reaction.
Examples
Show aldol condensation product:
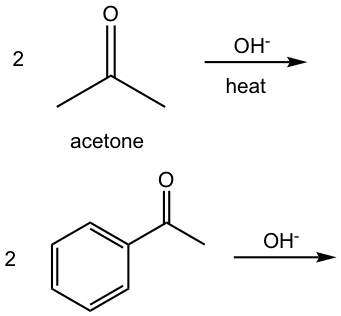
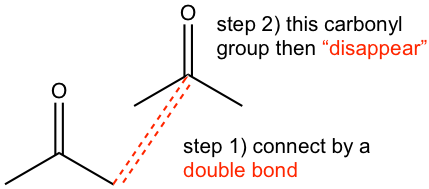
Strategy: (Take the first reaction as an example)
Answer:

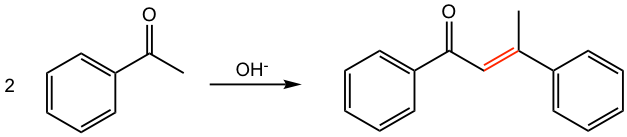
In the 2nd reaction here, a large extended conjugated system with two benzene rings is built with the formation of the new double bond. The special stability of the conjugated system makes the dehydration of this reaction occur easily and heating is not even necessary.
6.4.2 Crossed Aldol Reaction
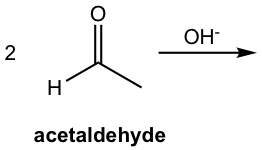
When two different carbonyl compounds, for example, A and B, are mixed together in an aldol reaction, four products could be formed statistically if no specific conditions are controlled. We will take the example of aldol condensation between acetaldehyde (A) and propanal (B) as an example. Both compounds can donate either enolate or the carbonyl group, so four possible products in total come from the combinations A-A, B-B, A-B, and B-A. The reactions from the combination of A-B or B-A are called crossed aldol reactions.
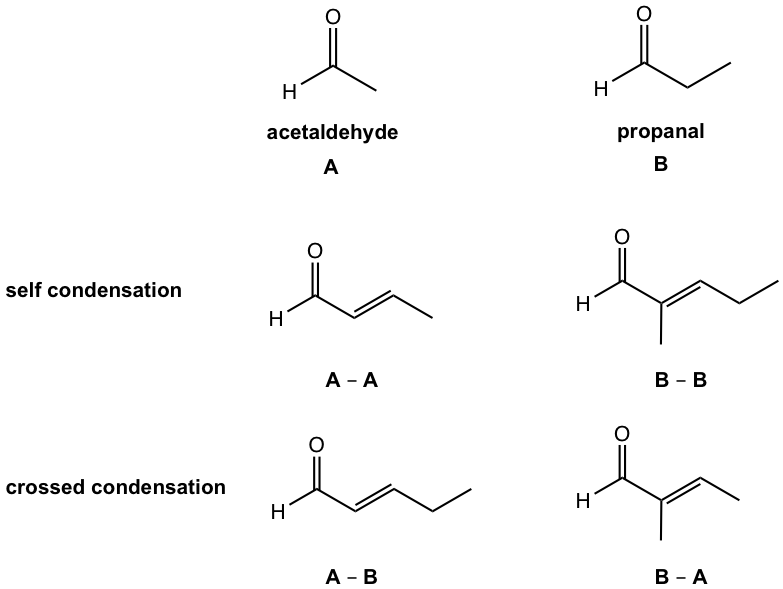
It is usually difficult to separate the four products apart since they have similar structures and similar physical properties, so the aldol reaction that produces four products is not useful for synthesis purposes.
On the other hand, if the structures of carbonyl compounds and reaction conditions are carefully controlled, a crossed aldol reaction could only produce one single product, it, therefore, could be used as a synthetic tool.
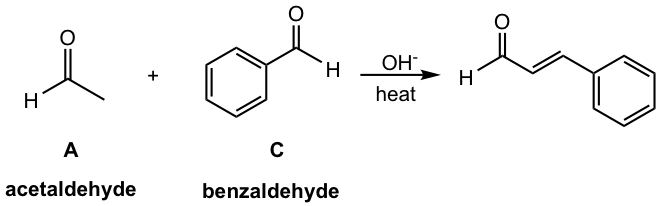
In the above reaction in Fig. 6.4g, only compound A (acetaldehyde) has an α-hydrogen, while compound C (benzaldehyde) does not have an α-hydrogen. Since C has no α-hydrogens, it cannot do self-condensation. The reaction is also carried out in a way that compound A is added slowly to a solution of compound C with base OH–. Since the concentration of compound A is always low under this condition, and it is present mostly in its enolate form, the possibility of self-condensation of compound A is also minimized. Therefore the reaction could only take place between the enolate of compound A and the carbonyl group of compound C, and produce the single product.
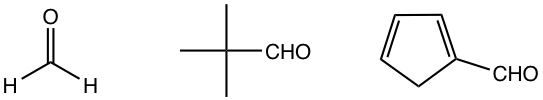
A couple of other carbonyl compounds with no α-hydrogen that are commonly used in crossed aldol reactions are listed in Fig. 6.4h.
6.4.3 Intramolecular Aldol Condensation
For a molecule that contains two carbonyl functional groups in the proper positions, the aldol condensation could take place intramolecularly to produce a cyclic product with five- or six-membered rings, and the reaction is also called aldol cyclization.
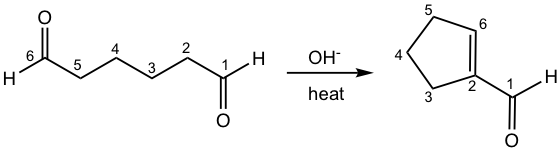
For the above reaction, one aldehyde provides enolate and the aldehyde on the other end provides the carbonyl group (as shown in the hint drawing). This example also represents a symmetric dialdehyde that only one product is possible for aldol cyclization.
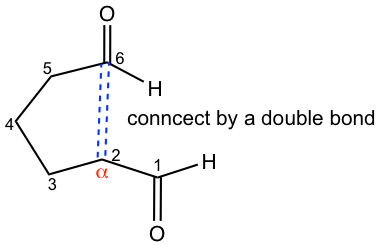
Let’s take a look at another example for the reaction of a keto aldehyde, and see how to decide the major product if multiple reaction pathways are available.
Examples
Show the product.
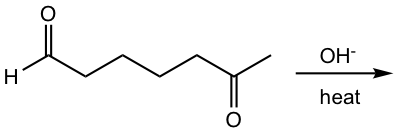
Analysis and answer:
Three different enolates are possible because there are two types of α-hydrogens on each side of ketone, and one type α-hydrogen of the aldehyde. However, the major product formed is the five-membered ring and this could be explained for two reasons:

- The carbonyl group in aldehyde shows a higher reactivity than that in ketone towards nucleophilic reaction, so the reaction takes place preferentially on the aldehyde carbonyl group.
- Five-membered rings form far more feasibly than seven-membered rings because of their stability, so the reaction occurs between the enolate from C-5 and the carbonyl group on C-1.

