Chapter 6: Reaction at the α-Carbon of Carbonyl Compounds
6.2 Halogenation of the α-Carbon
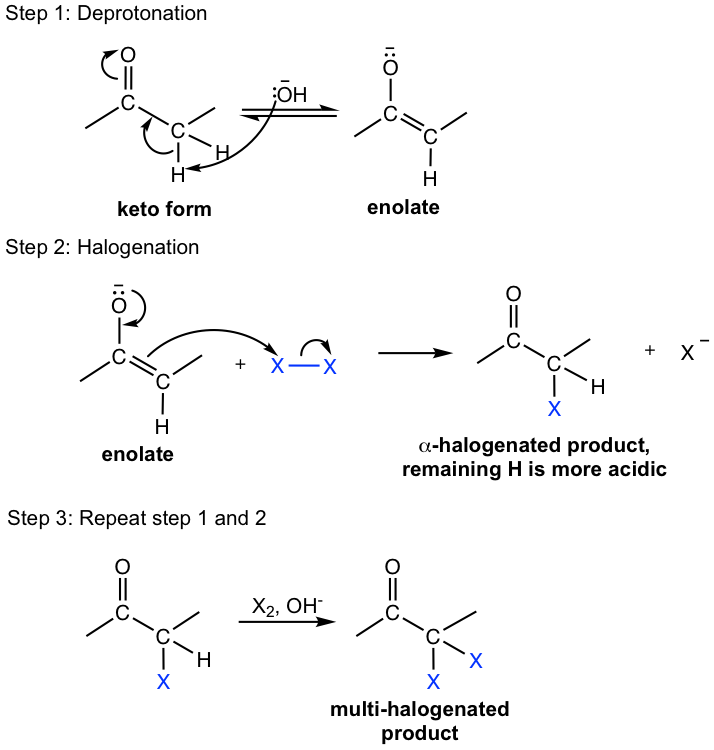
When halogen (Cl2, Br2, or I2) is added to the aldehyde or ketone, one or more of the α-hydrogens are replaced by halogens. The reaction proceeds with either acid or base present, through different reaction mechanisms.
Acid-Catalyzed Halogenation
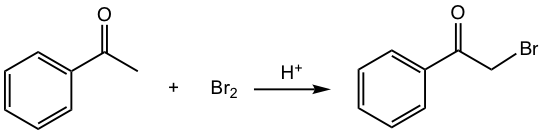
In acid-catalyzed halogenation, only one α-hydrogen is replaced by a halogen. The reaction mechanism (Fig. 6.2b) involves the formation of enol in acidic conditions in the first step, then the addition of halogen with enol followed by deprotonation.
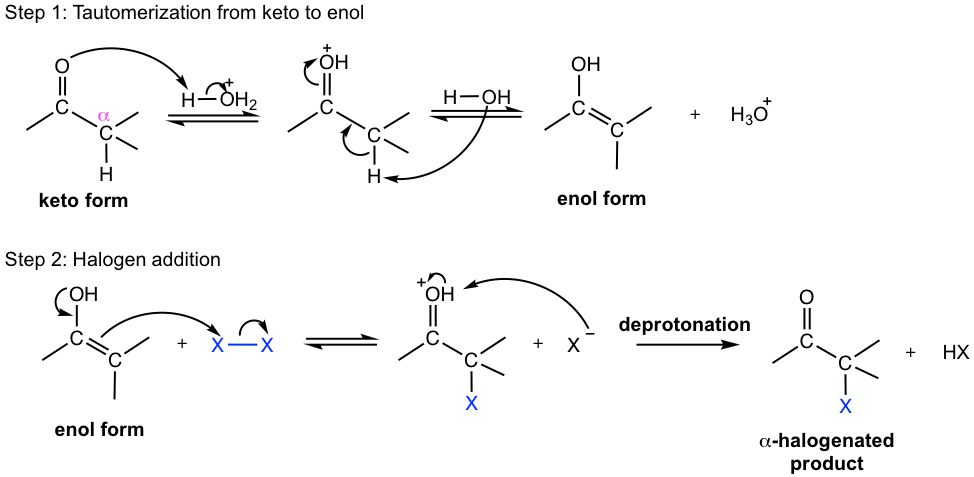
For α-halogenated aldehyde/ketone, because of the inductive electron-withdrawing effect of halogen, the electron density of the C=O group is lower than that in a regular aldehyde/ketone, so further protonation is not favored. Since protonation is the very first step in the mechanism, no further halogenation occurs. That explains why halogenation only takes place only once in acidic conditions.
Base-Promoted Halogenation
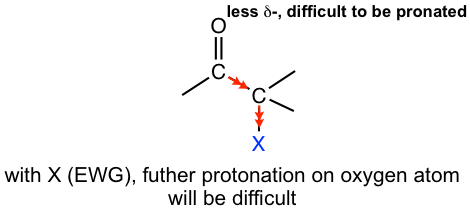
Under basic conditions, all of the α-hydrogens are replaced by excess halogens, with the halogenation process repeated multiple times.
This can be explained by the mechanism shown in Fig. 6.2e. The first step of halogenation is the deprotonation of the α-hydrogen, so enolate is generated. The enolate then reacts with halogen to produce the α-halogenated product. With the first halogen introduced in the structure, the inductive electron-withdrawing effect of halogen increases the acidity of the remaining α hydrogens, so the successive halogenations are more rapid and repeated more easily.
The Haloform Reaction
When the methyl ketone reacts with excess base and halogens, the carboxylate is produced as the final organic product. The side product of such a reaction is an inorganic compound called haloform, with the formula of CHX3, and as a result, the reaction is called a haloform reaction. When iodine is used, the bright yellow precipitate iodoform CHI3(s) is obtained. Iodoform reaction is usually used as a test for the presence of methyl ketone.
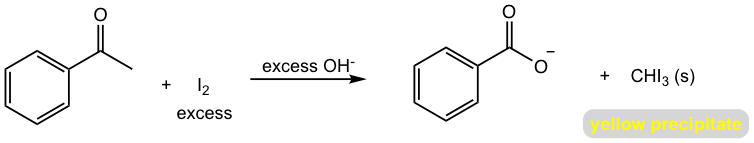
Other than being used for test purposes, the haloform reaction is also synthetically useful to prepare carboxylic acids from methyl ketones. The mechanism of haloform reaction is shown in Fig. 6.2g.
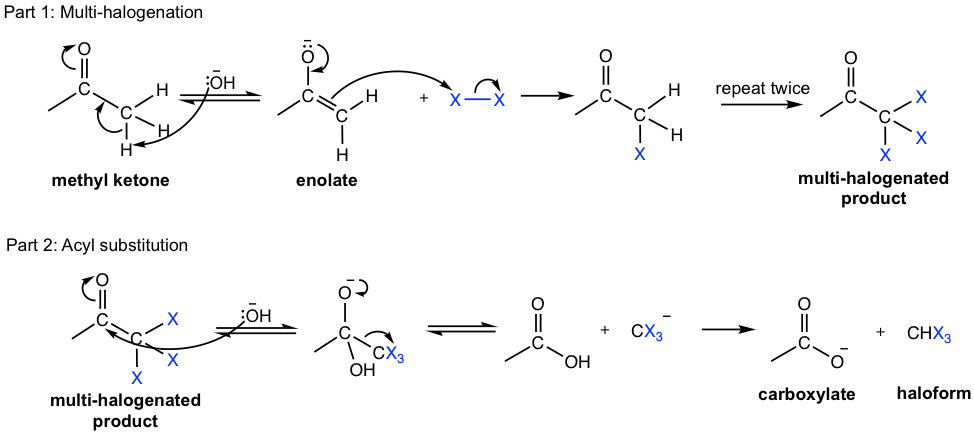
The first half of the haloform reaction is the same as halogenation under basic conditions, with all three α-hydrogens replaced by halogens. The resulting CX3 group bonded to the carbonyl can be a good leaving group and then replaced by OH– in the second half of the reaction, which leads to the formation of carboxylate salt and haloform.

