Chapter 5: More Reactions on Aromatic Compounds
5.6 Nucleophilic Aromatic Substitution: SNAr
The keyword for the reactions of aromatic systems we learned so far is electrophilic substitution. Here we will explore the possibility of nucleophilic substitution of aromatic compounds.
5.6.1 Nucleophilic Aromatic Substitution by Addition-Elimination (SNAr)
When we learned about nucleophilic substitution (SN1 or SN2), we saw that these reactions generally don’t apply to aryl halide.
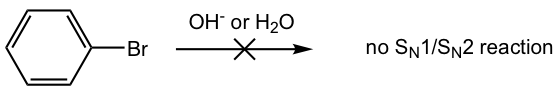
However, when there are one or more electron-withdrawing substituents present on aryl halide, it does undergo nucleophilic substitution, which involves the addition-elimination mechanism.
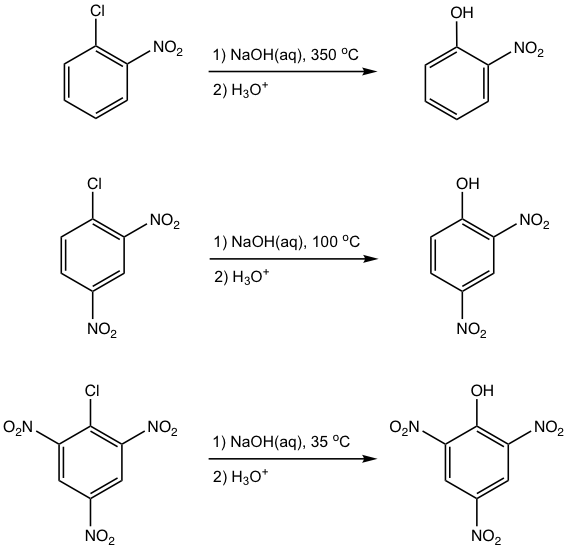
It is noticed from the above examples that the greater the number of electron-withdrawing substituents present, the more easily the nucleophilic aromatic substitution occurs (the lower the reaction temperature). Further studies also indicate that the electron-withdrawing substituent must be on ortho or para position to the halogen, to make the reaction possible. All these observations can be explained by the reaction mechanism that the electron-withdrawing substituents on ortho (or para) positions help to stabilize the carbanion intermediate (Fig. 5.6c).
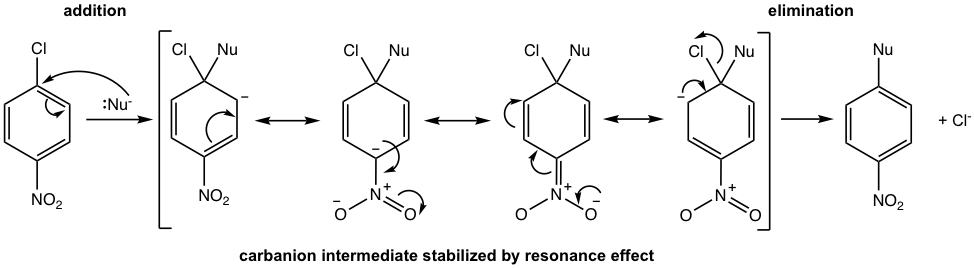
Such a mechanism is for nucleophilic aromatic substitution, a two-step process, with the symbol SNAr. In the first step, the nucleophile attacks the carbon attached to the leaving group (halide) and produces the carbanion intermediate. The negative charge of the carbanion can be stabilized via resonance effects of electron-withdrawing groups on ortho (or para) positions, as shown in Fig. 5.6c (only NO2 in the ortho position is shown as an example). The leaving group, which should also be a weaker base similar to the case in the SN2 reaction, is then eliminated in the second step, and the aromaticity is restored in the product.
5.6.2 Nucleophilic Aromatic Substitution that Forms a Benzyne Intermediate
Under harsh reaction conditions, aryl halide without electron-withdrawing substituents does react with strong nucleophiles for substitution. For example, when chlorobenzene is heated with NaOH solution at 350 °C, the Cl is replaced by OH, and phenol is obtained as a product.
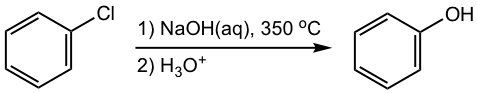
Chlorobenzene also reacts with amide (NH2–) and gives aniline product. When C-14 labeled chlorobenzene is treated with amide, half of the product has the amino group attached to the labeled carbon, and the other half has the amino group attached to the adjacent position of the labeled carbon, as shown in Fig. 5.6e. The C-14 labeled carbon is marked * in the reaction equation.

There are two unusual aspects of the above reactions:
- No electron-withdrawing group is present in the aryl halide.
- The amino group is attached to different carbons in the products.
This type of aromatic substitution has a different mechanism than SNAr (addition-elimination). It takes place through an elimination-addition mechanism that involves an unusual intermediate called benzyne, as shown in Fig. 5.6f.
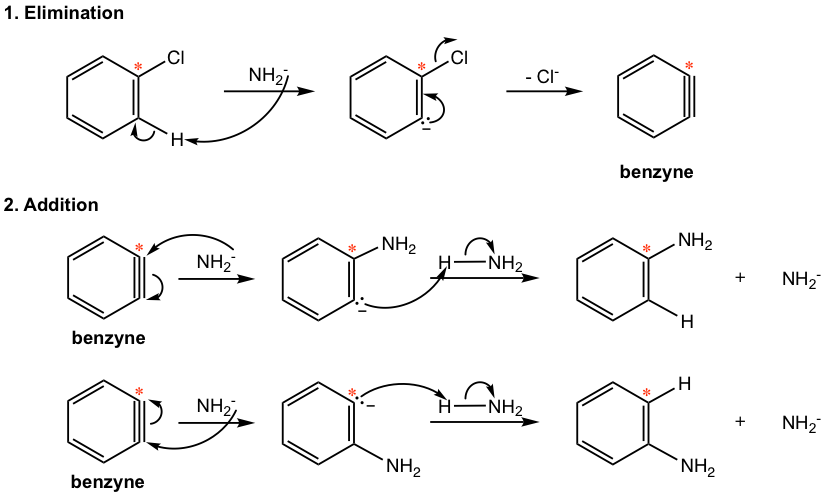
In the first part of the mechanism, HCl is eliminated and the benzyne intermediate is formed. The elimination of HCl involves two subsequent steps in which the hydrogen on the ortho position of chlorine is first abstracted by an amide ion, to produce the carbon anion, the anion then loses a chloride ion to form benzyne. The reaction of benzyne with nucleophile NH2–, followed by a protonation gives the final aniline product. When benzyne reacts with NH2–, the attack can occur on either carbon of the “triple bond”, which explains the formation of the two “isomers” with the NH2 group on different carbons.
The Structure and Chemistry of Benzyne
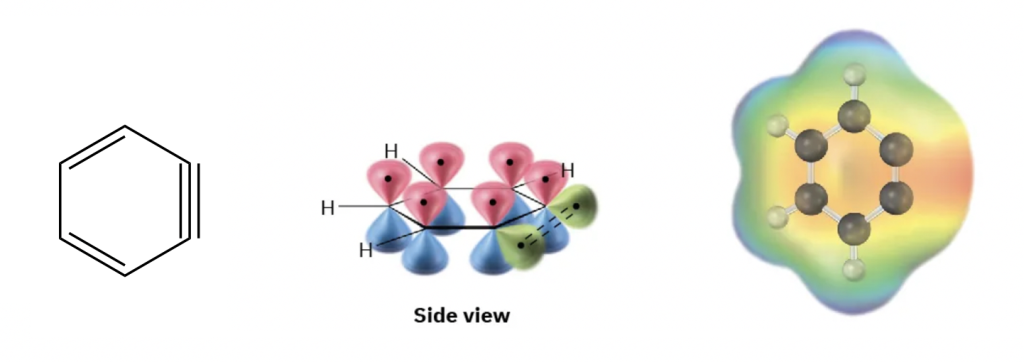
Benzyne is a highly unstable intermediate that has a “triple” bond. Although a triple bond is included in the structure formula of benzyne, it is not a regular triple bond. For a typical triple bond, both carbons are in sp hybridization, and the bond angle is 180°. The carbons in benzyne are in sp2 hybridization, however, and the bond angles are about 60°. Furthermore, the additional π bond in the benzyne is formed by sp2-sp2 overlapping, which is in the same plane of the ring and therefore very weak. As shown in Fig. 5.6g, benzyne is a distorted structure with great electron density on the triple bond edge and therefore is a highly unstable and reactive intermediate.
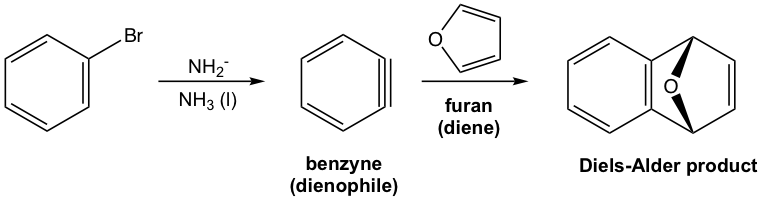
Benzyne is too unstable to be isolated. Other than the above reaction as evidence for the formation of benzyne intermediate, it can also be “trapped” by reacting with other appropriate reactants. When furan is added to the reaction that forms a benzyne intermediate, the Diels-Alder reaction product can be isolated that comes from the 4+2 addition of furan with benzyne.